ICU Management & Practice, Volume 21 - Issue 6, 2021

Nutrition Risk Screening
An assessment of nutrition risk is recommended by most guidelines, using validated tools combining anthropometric, clinical and lab data. The recommended tools to assess nutrition risk vary between guidelines, as a reflection of the differences of local practices.
In addition to the scores given by nutrition risk tools, extreme values of body mass index (BMI) are associated with increased morbidity and mortality. A J-shaped curve linking BMI with in-hospital mortality rate have been reported (Huang et al. 2020). Obesity is associated with a respiratory insufficiency likely exacerbated by a COVID-19 pneumonitis or respiratory complication that may increase the need for ventilatory support and hospital stay (Chetboun et al. 2021). The association between obesity and higher mortality has been reported by some (Gao et al. 2021; Du et al. 2021; Hendren et al. 2021), but not all investigators (Ullah et al. 2021; Deng et al. 2021). Interestingly, the abundance of ACE (angiotensin converting enzyme)-2 receptors in the adipose tissue can explain the high prevalence of severe forms of COVID-19 in obese patients, as a result of the prolonged residence of SARS-CoV-2. In particular, the amount of visceral fat has been reported as a risk factor for poor outcome (Huang et al. 2020; Földi et al. 2021). From a nutritional perspective, there is no reason to manage obese patients differently than non-obese. The adjusted weight is recommended to calculate the nutritional intakes (Singer et al. 2019).
A potential association between a low BMI and mortality of patients hospitalised for COVID-19 has been less extensively scrutinised (Caccialanza et al. 2021; Ochoa et al. 2020). Underweight and especially malnourished patients facing an acute inflammation such as COVID-19 can experience more frequent complications related to refeeding syndrome, muscle weakness and immune deficiency. Older age, male gender and chronic diseases (with or without inflammatory component) are known risk factors for both malnutrition (Cederholm et al. 2019), and poor outcome after a COVID-19 infection. Furthermore, a greater muscle mass is associated with successful extubation, shorter ICU length of stay and decreased hospital mortality (Damanti et al. 2021).
Nutrition Requirements and Prescriptions
The slow and progressive delivery of energy and protein is recommended by all guidelines, implying that the target should be achieved by 5-7 days after admission. A typical course of energy intake recorded during the ICU stay of a cohort of patients is displayed in Figure 2 (Hoyois et al. 2021).
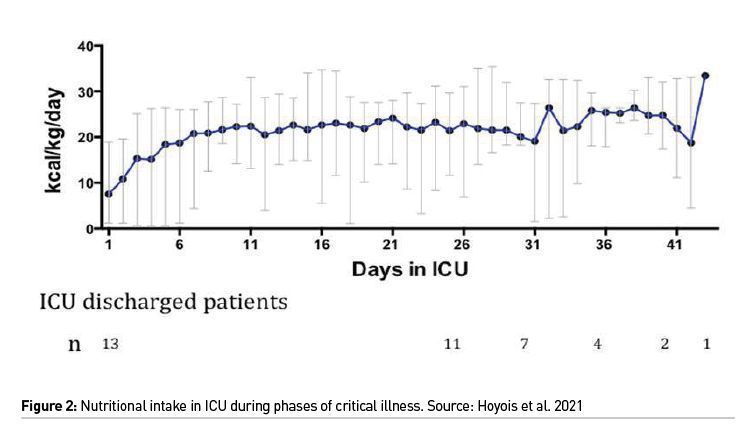
Energy
Persistent hypermetabolism reflected by high energy expenditure has been reported (Whittle et al. 2020; Niederer et al. 2021) during prolonged stays in an ICU, except during the period of muscle paralysis (Karayannis et al. 2021).
However, the measurement of energy expenditure by indirect calorimetry carries a risk of staff exposure to the virus, potential spread of disease, and/or workforce related demands. Hence, only one set of guidelines recommends that indirect calorimetry is used where safely available (Barazzoni et al. 2020), whereas three other sets of guidelines recommend against the use of indirect calorimetry (Martindale et al. 2020; Chapple et al. 2020; Campos et al. 2020). Most guidelines provide recommendations on the prescription of energy using a predictive equation. As high doses of sedative agents can be required in ventilated patients, the amount of non-nutritional calories from propofol and glucose administration can be high and these calories need to be considered in the calculation of energy balance, understanding that this is likely to negatively impact protein and micronutrient provision.
Proteins
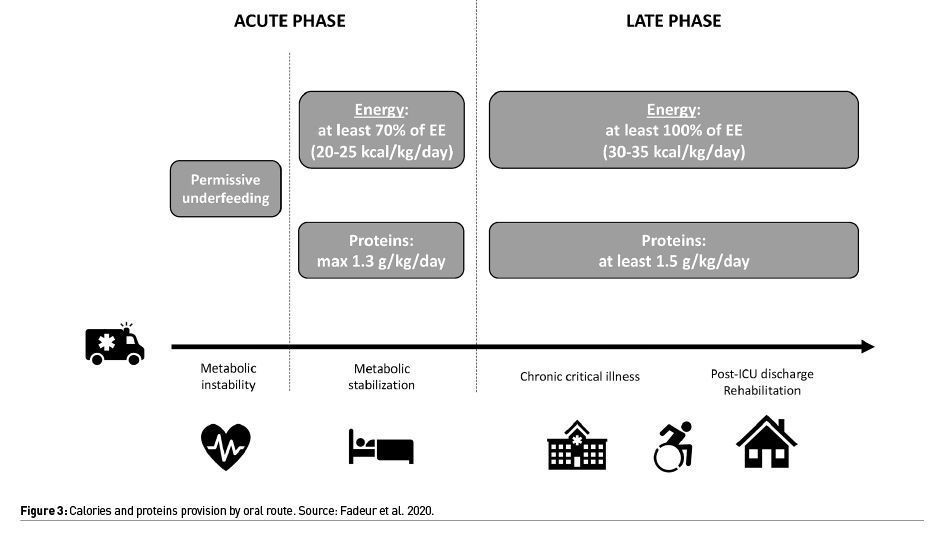
Most guidelines recommend using a high protein formula to achieve protein doses of 1.2-1.5 g/kg/day based on expert consensus and small observational studies in critically ill patients without COVID-19 (Figure 3) (Fadeur et al. 2020).
Micronutrients
Although not discussed in the available guidelines, there is a major enthusiasm for the use of vitamins and trace elements in patients with COVID-19 following the association of low vitamin C, D and zinc levels and poor outcomes. Interventional trials assessed the effects of high doses of these micronutrients. Overall, vitamin C and zinc supplementation did not improve the outcome, while oral vitamin D supplementation decreased the need for ICU admission, induced a faster negativity of SARS-CoV-2 tests and even mortality in one study (Speakman et al. 2021). This requires further investigation. At a minimum, micronutrients to the recommended dietary intakes should be provided and supplementation considered in patients who stay for extended periods or who have considerable nutrition inadequacy.
Timing of Initiation
Most guidelines recommend an early initiation of enteral nutrition within 48 h of admission, at least at a minimal trophic rate including in patients receiving low or decreasing doses of vasopressors.
Route of Feeding
Most guidelines recommend the enteral route (oral or enteral nutrition (EN)) in preference of parenteral nutrition (PN) for nutrition therapy, via the nasogastric (NG) route.
The delivery of EN can be impaired by gastrointestinal (GI) dysfunction during the ICU stay (Hoyois et al. 2021). The limited tolerance to EN can be related to a dysfunction of GI tract, reflected by the high prevalence of GI symptoms upon admission or during the ICU stay in COVID-19 patients (Kariyawasam et al. 2021; Blaser et al. 2021a; Drake et al. 2021) and frequent GI complications reported. Moreover, the significant need for NMB agents and prone positioning to aid ventilation can also impair GI intolerance. Notably, the presence of GI symptoms has been associated with higher illness severity, reflected in a higher need for hospital admission, ICU admission and intubation, even after adjustment for demographics, comorbidities, and other clinical symptoms (Bishehsari et al. 2021). Cytotoxic enterocyte injury and microvascular injury and thromboinflammation have been incriminated (Blaser et al. 2021a). Hence, EN should be withheld and gradually recommenced upon patient stabilisation in the case of uncontrolled shock and haemodynamic instability.
Mode of Feeding
Six guidelines recommend continuous
EN, as this mode is easier to manage and could be associated with a lower risk of diarrhoea and less frequent patient interaction for staff with continuous EN, decreasing exposure of healthcare professionals to COVID-19.
Monitoring
Gastric residual volumes
Seven guidelines make recommendations around the use of gastric residual volumes (GRVs) to monitor EN tolerance using cut-off values of 300-500 ml. However, as acknowledged in most guidelines,
the routine monitoring of GRVs may be unreliable to detect delayed gastric emptying, can impact nutrition delivery if EN is stopped or reduced unnecessarily, and may be a risk of viral transmission to the healthcare provider. In the absence of GRV measurement, other clinical features of EN intolerance including abdominal distension and regurgitation should be closely monitored (Blaser et al. 2021b).
Electrolytes
Four guidelines mention that re-feeding syndrome risk should be considered in critically ill patients admitted with COVID-19, as poor appetite and intake and GI symptoms are common prior to and during hospital admission. These guidelines recommend close monitoring and replacement of potassium, phosphate, and magnesium when commencing nutrition support (Boot et al. 2018).
Nutrition adequacy
To prevent under- or over-feeding, the majority of guidelines recommend close monitoring of nutrition adequacy (energy and protein delivery compared to estimated or measured requirements). This is particularly true in the setting of a pandemic where normal models of care are disrupted and the risk for nutrition failure is significant.
Specific Patient Populations and Conditions
Prone positioning
Early and continuous EN is recommended, while patients are in the prone position. Patients in the prone position may have increased GI intolerance, and prokinetics and insertion of post-pyloric tubes should be considered as necessary. PN may be needed in cases of significant EN intolerance and nutrition deficit.
Extracorporeal membrane oxygenation
Three guidelines make specific recommendations for patients receiving extracorporeal membrane oxygenation (ECMO) including early EN in this patient group. Patients on ECMO are likely to have high metabolic needs (e.g. after ICU day 5 up to 30 kcal/kg and 1.5–2 g protein/kg day in normal-weight individuals).
Non-intubated critically ill patients
Seven guidelines make recommendations for non-intubated patients. The overarching theme is that these patients are at high nutrition risk and that a high energy and high protein diet and oral nutrition supplements should be provided. Escalation to EN should occur if energy and protein intakes are inadequate (e.g. meeting <50–65% targets after 5 days). Some guidelines specifically recommend avoiding early removal of NG tubes post extubation.
Equipment Considerations
Two guidelines recommend assessment of equipment needs early and development of plans in the event of equipment (e.g. feeding pumps and delivery lines) and nutrition formula shortages.
Workforce Recommendations
Two guidelines make specific recommendations regarding dietetic workforce capacity during the COVID-19 pandemic. This includes rapidly identifying additional staff who could be upskilled in the case of significant admission numbers or staff sickness (such as the use of appropriately trained allied health assistant staff, or training of dietetic staff who have transferable skills in specific ICU nutrition processes). It is also recommended that training be commenced early with an appropriate education package that has been developed by an experienced critical care dietitian, and that the most experienced critical care dietitians see the sickest patients. Five guidelines mention the use of remote working processes to protect staff from infection risk of COVID-19.
In summary, most available recommendations do not differ from guidelines for the management of general ICU patients although the risk of contamination supports the avoidance of unnecessary invasive manipulation. The level of evidence supporting these guidelines is very low in the absence of large-scale prospective studies and the appropriateness of the recommendations needs to be considered in the context it is being applied.
Conflict of Interest
None.
References:
Barazzoni R, Bischoff SC, Breda J et al. (2020) ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr, 39 :1631-1638
Bishehsari F, Adnan D, Deshmukh A et al. (2021) Gastrointestinal symptoms predict the outcomes from COVID-19 infection. J Clin Gastroenterol.
Blaser AR, Gunst J, Arabi YM (2021a) The gut in COVID-19. Intensive Care Med, 47:1024-1027.
Blaser AR, Deane AM, Preiser JC et al. (2021b) Enteral Feeding Intolerance: Updates in Definitions and Pathophysiology. Nutr Clin Pract, 36:40-49.
Boot R, Koekkoek KWAC, van Zanten ARH (2018) Refeeding syndrome: relevance for the critically ill patient. CurrOpin Crit Care, 24:235-240.
Caccialanza R, Formisano E, Klersy C et al. (2021) Nutritional parameters associated with prognosis in non-critically ill hospitalized COVID-19 patients: The NUTRI-COVID19 study. Clin Nutr.
Campos LF, Barreto PA, Duprat G et al. (2020) BRASPEN’s nutritional statement for coping with COVID-19 in hospitalized patients. Supported by Brazilian Intensive Care Medicine Association. Version 23.
Cederholm T, Jensen GL, Correia MITD et al. (2019) GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. Clin Nutr, 38:1–9.
Chapple LA, Tatucu-Babet OA, Lambell KJ et al. (2021) Nutrition guidelines for critically ill adults admitted with COVID-19: Is there consensus? Clin Nutr ESPEN, 44 :69-77.
Chapple LS, Fetterplace K, Asrani V et al. (2020) Nutrition management for critically and acutely unwell hospitalised patients with coronavirus disease 2019 (COVID-19) in Australia and New Zealand. Aust Crit Care, 33 :399-406.
Chetboun M, Raverdy V, Labreuche J et al. (2021) BMI and pneumonia outcomes in critically ill COVID‐19 patients: an international multicenter study. Obesity, 29:1477-1486.
Drake TM, Riad AM, Fairfield CJ et al. (2021) Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study. Lancet, 398:223-237.
Damanti S, Cristel G, Ramirez GA et al. (2021) Influence of reduced muscle mass and quality on ventilatorweaning and complications during intensive care unit stay in COVID-19 patients. Clinical Nutrition.
Deng L, Zhang J, Wang M, Chen L (2021) Obesity is associated with severe COVID-19 but not death: a dose−response meta-analysis. Epidemiol Infect, 149.
Du Y, Lv Y, Zha W et al. (2021) Association of body mass index (BMI) with critical COVID-19 and in-hospital mortality: A dose-response meta-analysis. Metabolism, 117.
Fadeur M, Preiser JC, Verbrugge AM et al. (2020) Oral Nutrition during and after Critical Illness: SPICES for Quality of Care! Nutrients, 12:3509.
Földi M, Farkas N, Kiss S et al. (2021) Visceral Adiposity Elevates the Risk of Critical Condition in COVID-19: A Systematic Review and Meta-Analysis. Obesity, 29:521-528.
Gao M, Piernas C, Astbury NM et al. (2021) Associations between body-mass index and COVID-19 severity in 6·9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol, 9:350–9.
Hendren NS, de Lemos JA, Ayers C et al. (2021) Association of Body Mass Index and Age With Morbidity and Mortality in Patients Hospitalized With COVID-19: Results From the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation, 143:135–44.
Hoyois A, Ballarin A, Thomas J et al. (2021) Nutrition evaluation and management of critically ill patients with COVID-19 during post-intensive care rehabilitation. J Parenter Enteral Nutr, 45:1153-1163.
Huang Y, Lu Y, Huang YM et al. (2020) Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism, 113:154378.
Huang HK, Bukhari K, Chiung-Hui PC et al. (2021) The J-shaped relationship between body mass index and mortality in patients with COVID-19: A dose-response meta-analysis. Diabetes ObesMetab, 23:1701–9.
Karayiannis D, Maragkouti A, MikropoulosT et al. (2021) Neuromuscular blockade administration is associated with altered energy expenditure in critically ill intubated patients with COVID-19. Clin Nutr, 25;S0261-5614.
Kariyawasam JC, Jayarajah U, Riza R et al. (2021) Gastrointestinal manifestations in COVID-19. Trans R Soc Trop Med Hyg.
Martindale R, Patel JJ, Taylor B et al. (2020) Nutrition therapy in the patient with COVID-19 disease requiring ICU care. American Society for Parenteral and Enteral Nutrition.
Minnelli N, Gibbs L, Larrivee J, Sahu KK (2020) Challenges of Maintaining Optimal Nutrition Status in COVID-19 Patients in Intensive Care Settings. J Parenter Enteral Nutr, 44:1439–1446.
Niederer LE, Miller H, Haines KL et al. (2021) Prolonged progressive hypermetabolism during COVID-19 hospitalization undetected by common predictive energy equations. Clin Nutr ESPEN, 45: 341–350.
Ochoa JB, Cárdenas D, Goiburu ME et al. (2020) Lessons Learned in Nutrition Therapy in Patients With Severe COVID‐19. J Parenter Enter Nutr, 44.
Singer P, Blaser AR, Berger MM et al. (2019) ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr, 38:48-79.
Speakman LL, Michienzi SM, Badowski ME (2021) Vitamins, supplements and COVID-19 : a review of currently available evidence. Drugs Context, 10 :2021-6-2.
Ullah W, Roomi S, Nadeem N et al. (2021) Impact of Body Mass Index on COVID-19-Related In-Hospital Outcomes and Mortality. J Clin Med Res, 13.









