ICU Management & Practice, Volume 22 - Issue 1, 2022
Plasma volume (PV) is the level of intravascular fluid minus the red blood cells, white blood cells and platelets. PV in heart failure (HF) patients is associated with increases in fluid compartments. For example, PV could increase by nearly 40% in patients with decompensated HF (Kobayashi et al. 2021). PV can also expand due to accumulated fluid volume leading to impaired pulmonary circulation and hospitalisation. Therefore, a reliable assessment of PV is essential in HF patients. Haemoconcentration is typically determined by a change in haemoglobin or haematocrit concentrations. This can function as an indirect marker of changes in PV.
Estimated plasma volume (ePVS), derived from haemoglobin and haematocrit, has also been shown to have an association with other congestion biomarkers (p.e. E/e’ measured in echocardiography). It is a useful diagnostic and prognostic tool in HF management. Elevated ePVS has been repeatedly shown to be associated with clinical outcomes in patients with acute or chronic HF.
Formulas to Estimate ePVS
Two formulas estimate PV.
The first formula was initially proposed by Strauss to estimate changes in PV, solely using haemoglobin and haematocrit.
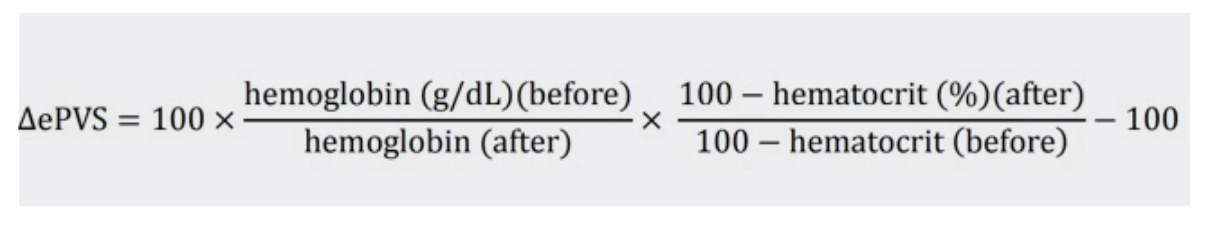
An extension of this formula was first published by Duarte et al. It provides an instantaneous measurement of PV using haematocrit and haemoglobin data from a single time-point.

The second formula, the Kaplan/Hakim formula, is also used to calculate actual and ideal PV using haematocrit and dry body weight.

These formulas both were reported to predict clinical outcomes in patients with HF. However, in clinical practice, ePVS estimates from Duarte formula represent an easy-to-use tool as it only relies on haemoglobin and haematocrit (and not on dry body weight – something that is difficult to assess in acute settings). An association between classical congestion markers and ePVS has been reported only with the Duarte formula, possibly because the Hakim formula incorporates dry body weight. ePVS derived from the Duarte formula has been reported in a haemodynamic study to be a marker of left-sided haemodynamic congestion. These data suggest that ePVS estimation using the Duarte formula could become a useful congestion marker in the management of HF, but there is a need for large-scale multicentre studies to ascertain the clinical usefulness of ePVS in HF patients (Kobayashi et al. 2021).
There is some controversy as to what ePVS actually measures. Indeed, in some studies, ePVS derived from haemoglobin and haematocrit were sizably different from calculated PV for isotope. The answer is unsettled. However, it is important to note that the methods may not necessarily be measuring the same variable. By essence, ePVS based on haemoglobin and haematocrit are instantaneous estimates, whereas isotopes based method may provide more steady estimates. These differences in timings may be the cause for the variation. Importantly, regardless of these discrepancies, ePVS undeniably has an important prognostic value in the field of HF and HF and can provide a phenotypic characteristic. This can provide clinicians the opportunity to tailor personalised therapy for patients with HF (Kobayashi et al. 2021).
Congestion and ePVS
Congestion is a well-known predictor of outcome in patients with HF, including higher rates of readmission and death. Congestion at the time of admission and residual congestion are both associated with poor clinical outcomes and a major cause for HF hospitalisation (Tamaki et al. 2019). Despite this clearly established association, HF patients are often discharged with clear symptoms of congestion without a pre-discharge clinical assessment. It is important to detect and monitor congestion before it progresses to decompensation. Similarly, post-discharge assessment of congestion is also not a matter of routine practice.
This contributes to increased cost and a higher burden of rehospitalisation. An evaluation of physical symptoms, laboratory reports and net fluid change should be considered part of a pre-discharge assessment. ePVS data is usually available but is rarely looked at despite evidence that it could be associated with improvement in patient outcomes. This is especially true since evaluating congestion in heart failure with reduced ejection fraction (HFrEF) can be difficult.
There is a need for clinicians to identify and use approaches that could improve the management of congestion to prevent readmissions and improve patient outcomes.
ePVS and Acute and Chronic HF
A post-analysis of the EPHESUS (Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study) first showed that in patients with HF and left ventricular systolic dysfunction complicating acute myocardial infarction (AMI), a short-term decrease in ePVS using the Strauss formula is associated with better cardiovascular outcomes. In addition, an instantaneous estimation of PV derived from the Strauss formula reported a greater prognostic value (Duarte et al. 2015). Findings from the EPHESUS have been tested and validated in a wide range of other heart failure settings, whether it is acute, chronic, heart failure with preserved ejection fraction (HFpEF) or heart failure with reduced ejection fraction (HFrEF). ePVS and its association with clinical outcomes has also been reported in patients with chronic HF.Patients with acute heart failure have more interstitial fluid volume. Several reports have found an association between an increase in ePVS and a higher risk of clinical outcomes. Increased ePVS at discharge has been found to be associated with poor prognosis. Hence, the clinical and prognostic value of ePVS at discharge and post-discharge can be valuable. While this may be challenging, it can help optimise patient management and prevent hospital readmissions (Kobayashi et al. 2021).
In a recent study by Chen et al. (2021), higher ePVS calculated from the Duarte formula was found to be associated with poor prognosis in patients with AMI. The main findings from the MIMIC-II study show that a higher level of ePVS is independently associated with a higher risk of in-hospital death of patients and that patients with higher-level ePVS have a lower possibility of survival during hospitalisation compared with patients with a lower-level ePVS.
Overall, it is important to recognise the clinical and prognostic value of ePVS and a careful pre – and post-discharge congestion assessment. Estimation of PV with the Strauss formula or Duarte formula can be a useful strategy and can have important clinical implications for patient management and improved patient outcomes. It should be assessed and closely monitored, and this can be done through serial measurements of ePVS using either blood count or body weight. ePVS has been repeatedly shown to be associated with outcome, even in patients hospitalized for AMI. However, ePVS estimation remains probably an underused strategy even though it can be useful to guide treatment strategies in patients with HF. Yet, we still need large-scale outcome clinical trial to clarify the impact of ePVS-guided management in patients with HF.
Key Points
- Estimated plasma volume (ePVS) is a useful diagnostic and prognostic tool in heart failure (HF) management.
- Elevated ePVS can be an important predictor of all-cause mortality in patients with HF.
- The Strauss formula and Duarte formula are routinely used to estimate plasma volume.
- ePVS enables repeated (and unexpensive) evaluations of congestion.
- Large-scale outcome clinical trials are needed to determine whether ePVS-guided management should become the standard of care in patients with HF.
For more details visit https://iii.hm/1eiy
Disclaimer
Point-of-View articles are the sole opinion of the author(s) and they are part of the ICU Management & Practice Corporate Engagement or Educational Community Programme.
References:
Chen J, Shen J, Cai D et al. (2021) Estimated plasma volume status (ePVS) is a predictor for acute myocardial infarction in-hospital mortality: analysis based on MIMIC-III database. BMC Cardiovasc Disord. 21, 530.
Duarte K, Monnez JM, Albuisson E et al. (2015) Prognostic Value of Estimated Plasma Volume in Heart Failure. JACC Heart Fail. 3(11):886-93.
Kobayashi M, Girerd N, Duarte K et al. (2021) Estimated plasma volume status in heart failure: clinical implications and future directions. Clin Res Cardiol. 110(8):1159-1172.
Tamaki S, Yamada T, Morita T et al. (2019) Prognostic Value of Calculated Plasma Volume Status in Patients Admitted for Acute Decompensated Heart Failure - A Prospective Comparative Study With Other Indices of Plasma Volume. Circ Rep. 1(9):361-371.







