ICU Management & Practice, Volume 23 - Issue 4, 2023
In this article, we will examine clinical concepts that have persisted over time, despite advancements in our understanding of physiology and technological innovations that have demonstrated their inapplicability in the routine clinical care of paediatric patients requiring respiratory support. These enduring beliefs have effectively transformed into myths.
Background
Invasive mechanical ventilation (IMV) in paediatric and neonatal patients displays heterogeneity concerning ventilator programming and patient monitoring. Despite the presence of guidelines outlining management principles that have demonstrated a reduction in mortality among paediatric and neonatal patients under mechanical ventilation, these guidelines are not universally adopted by clinicians (Bhalla et al. 2021). Furthermore, in developing countries, myths and suboptimal practices surrounding IMV are prevalent due in large part to a scarcity of specialists and insufficient education in patient management (Zenteno et al. 2021). This situation can lead to prolonged hospital stays and readmissions. Additionally, the literature on this topic is sparse compared to studies involving adult patients on IMV, leaving significant gaps in the current body of knowledge. Therefore, we conducted a review focusing on seven of these myths surrounding paediatric and neonatal IMV.
Myth 1: The use of cuffed endotracheal tubes is not recommended for paediatric patients
Historically, uncuffed endotracheal tubes (ETTs) were preferred for young children because the paediatric airway narrows below the vocal cords, creating an anatomical seal around the distal tube. Concerns about tracheal injury associated with cuff usage, such as fistulas or tracheal stenosis, led to the use of uncuffed ETTs for an extended period. The incidence of tracheal injuries from ETTs decreased with the introduction of low-pressure cuffs in the 1970s (Stoller 1999). Prior to 2010, both cuffed and uncuffed ETTs were deemed acceptable for intubating infants and children. Cuffed devices were recommended in specific clinical scenarios, such as low lung compliance, high airway resistance, or glottic air leaks (Kleinman et al. 2010).
Numerous contemporary studies and systematic reviews now support the safety of cuffed ETTs (De Orange et al. 2017; Chen et al. 2018; Shi et al. 2016). Advantages of using cuffed ETTs include improved capnography accuracy, reduced need for tube changes (which can lead to high-risk reintubations or delayed compressions), potential decrease in aspiration risk, and improved administration (and measurement) of pressure and tidal volume during mechanical ventilation—an essential aspect of preventing ventilator-induced lung injury (Chambers et al. 2018; Schweiger et al. 2013; Weiss et al. 2009). Subglottic stenosis is rare when employing cuffed ETTs in children and following meticulous technique (Black et al. 1990). European and North American paediatric cardiopulmonary resuscitation guidelines advocate for the use of cuffed ETTs in paediatrics (Topjian et al. 2020; Van de Voorde et al. 2021). It is vital to monitor cuff pressure and adhere to each manufacturer's recommendations (typically <20 to 25 cm H2O). Cuff pressures are dynamic during transport at altitude (Orsborn et al. 2016) and with increasing airway oedema.
The smallest internal diameter available for Microcuff© ETTs is 3.0 mm, recommended solely for newborns ≥3 kg. No standardised guideline exists for proper cuff management or methods to determine inflation volume, maximum pressure usage, and frequency of pressure measurements in neonates. Comparative studies between cuffed and uncuffed ETTs in the neonatal population were absent until 2016 (Thomas et al. 2016). A pilot study published in 2019 with 76 patients compared Microcuff© ETTs with and without cuffs in neonates > 35-week gestation up to 3-month-old infants weighing ≥3 kg and requiring ventilation management in the neonatal and paediatric intensive care unit. The uncuffed ETT group exhibited significantly higher rates of re-intubation at any point during the ventilation period and more frequent episodes of atelectasis or other ventilation-related complications. No differences were observed in postextubation stridor rates, post-extubation dexamethasone use, nebulised adrenaline post-extubation, or reintubation due to airway obstruction. At 34.7 months of follow-up, none of the patients in either group had developed subglottic stenosis. Caution was exercised in recommending confirmation of these results through larger multicentre studies (Thomas et al. 2019).
Myth 2: Patients weighing less than 10 kg should be ventilated in pressure-controlled assist-control mode, while those weighing more than 10 kg should be ventilated in volume-controlled assist-control mode
The choice of ventilatory mode in paediatric patients has been historically influenced by a paradigm: using pressure-controlled assist-control ventilation (PC-AC) for patients weighing less than 10 kg and neonates, and volume-controlled assistcontrol ventilation (VC-AC) for those weighing over 10 kg. This was attributed to the following reasons: 1) the belief that VC-AC mode lacked continuous flow and significant leaks from an uncuffed endotracheal tube would interfere with ventilation; 2) the inability to compensate for compressible volume in the airway; and 3) technical limitations of some ventilators to provide the required low tidal volumes (Vt) and flows to prevent volutrauma (Gregory et al. 1971). These largely technical challenges have been addressed with the advent of ventilators and circuits adapted for paediatric and neonatal ventilation.
The proximal flow sensor can accurately measure tidal volume and low flows, and these ventilators can deliver tidal volume and low flows while compensating for compressible volume and leaks, providing continuous basal flow.
The advantages of ventilating patients weighing less than 10 kg in VC-AC mode include strict control over tidal volume, potentially avoiding volutrauma, closer monitoring of plateau pressure (Pplateau) and driving pressure (DP), as well as improved alveolar air distribution, resulting in more homogeneous distribution and reduced risk of barotrauma and pneumothorax. The main limitation of VC-AC mode is the lack of consensus on determining the optimal protective tidal volume formula for the paediatric population. An alternative is the use of pressure-regulated volume control mode (PRVC) in premature newborns, which has shown benefits in survival and prevention of volutrauma in bronchopulmonary dysplasia.
A systematic review and meta-analysis in 2017 identified 20 controlled and randomised studies comparing VolumeControlled Ventilation (VCV) and PressureLimited Ventilation (PLV) in neonates and premature neonates (Klingenberg et al. 2017). VCV compared to PLV resulted in:
- Shorter duration of mechanical ventilation by 1.35 days.
- Lower incidence of pneumothorax.
- Lower incidence of bronchopulmonary dysplasia (BPD) at 36 corrected weeks.
- Lower incidence of periventricular leukomalacia or grade 3 or 4 intraventricular haemorrhage.
- A non-significant trend towards lower mortality.
Myth 3: Low PEEP levels of 0 to 4 cm H2O should be programmed for paediatric and neonatal patients.
Lung volume changes occur only when changes in transpulmonary pressure (PTP) magnitude occur. Contrary to intuition, lung volume change is not solely influenced by the value of alveolar pressure (Palv) but rather by the value of PTP. As the lung fills with air, each lung volume corresponds to a specific PTP value (Medina 2015).
The summary of the aforementioned is depicted in Table 1. Regardless of the values of Palv and pleural pressure (Ppl), if PTP is +5 cmH2O, the lung fills with a volume of air corresponding to functional residual capacity (FRC). If PTP = +30 cmH2O, the lung volume matches total lung capacity (TLC). And if PTP = +3 cmH2O, the corresponding volume is residual volume (RV). The penultimate row in the table represents a scenario where the patient has pleural effusion, causing the intrapleural pressure to become positive (+5 cmH2O). Without applying PEEP of +10 cmH2O for ventilation, their end-expiratory volume wouldn't reach FRC.
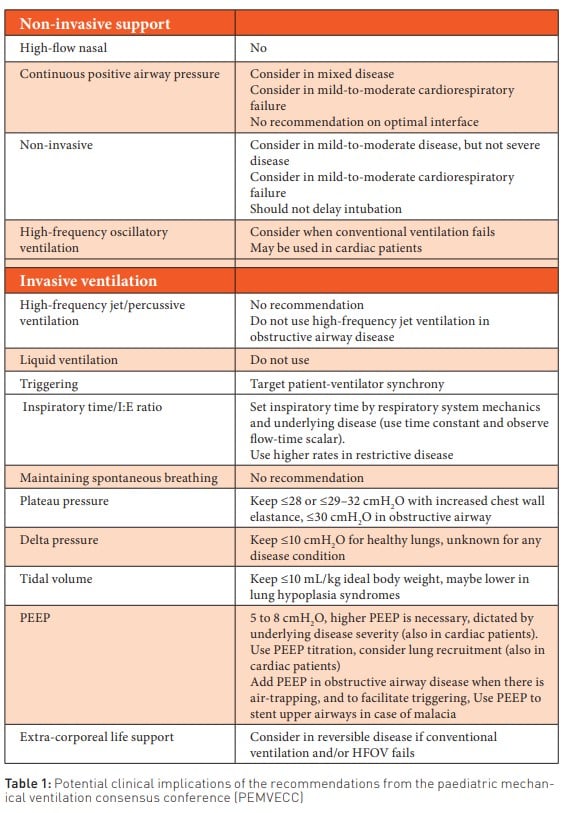
It has been suggested that PEEP ranges of 5-8 cmH2O are necessary during invasive mechanical ventilation, and higher PEEP levels may be necessary based on the severity of the underlying disease (also in cardiac patients). Adjusting PEEP levels should always be considered, including adding PEEP in obstructive lung disease when air trapping is present. In cases of malacia, PEEP is used to place a stent in the upper airways (Kneyber et al. 2017).
Myth 4: The synchronised intermittent mandatory ventilation (SIMV) mode should be used for weaning off the mechanical ventilator
MV improves survival in patients with respiratory failure, yet this therapy is not without complications, including ventilator-induced lung injury (VILI), ventilator-associated pneumonia, critical illness-associated weakness, right ventricular dysfunction, and increased costs associated with prolonged MV. Consequently, ventilator withdrawal should occur as soon as the patient is capable of maintaining adequate spontaneous breathing.
Ventilator withdrawal encompasses two scenarios: the gradual reduction of respiratory support (weaning) and the removal of the endotracheal tube (extubation). Extubation failure (EF) refers to a set of conditions leading to reintubation and VM reestablishment within the first 72 hours post-extubation. The decision to initiate weaning depends on the fulfilment of specific clinical criteria, including control of the underlying cause necessitating intubation and MV, effective gas exchange, appropriate neuromuscular condition, sufficient consciousness to protect the airway, and stable haemodynamic status.
The most commonly used weaning method in paediatrics involves the synchronised intermittent mandatory ventilation (SIMV) mode. This mode is often programmed with pressure support to achieve a target tidal volume (Vt) based on patient needs. The theoretical advantage is to alleviate additional respiratory effort imposed by the endotracheal tube and mechanical ventilator circuit. However, in adults, it is evident that this method significantly prolongs MV compared to daily spontaneous breathing trials (SBT) and pressure support ventilation (PSV). Therefore, its use is not recommended.
Commonly employed SBT methods include continuous positive airway pressure (CPAP), tube T trials, and PSV. In paediatrics, method choice largely depends on the treating team's experience, as there is no conclusive evidence that one method is superior to another. Implementing a ventilator withdrawal protocol that includes SBT allows for early identification of patients ready for weaning and facilitates a safer withdrawal process.
Myth 5: PSV is ineffective in paediatrics due to children becoming fatigued
In 2001, evidence-based guidelines were published for weaning and discontinuing ventilatory support. They classified adult studies on weaning from MV into 1) discontinuation assessment trial (ERT) strategies, 2) controlled trials of gradual reduction in mechanical support, and 3) controlled trials of alternative discontinuation strategies.
A study by Esteban et al. (1997) compared 2-hour spontaneous breathing trials with PS of 7 cmH2O to tube T trials. A higher number of patients in the PS group tolerated the trial and were extubated at the end of the study compared to the tube T group (86% vs. 78%; relative risk of failure, 0.64; 95% CI, 0.43 to 0.94). There was no difference in reintubation rates. A similar second study by Esteban et al. (1999) also showed no differences in reintubation rates between the groups. However, the shorter tube T trial benefited patients by reducing ICU and hospital stays (2 and 5 days shorter, respectively).
Five randomised clinical trials compared alternative methods to reduce ventilatory support in patients where several days of extubation were thought to be needed. The most informative results came from the two largest studies by Esteban et al. (1997) and Brochard et al. (1994). Both showed that when patients were initially evaluated for extubation using a tube T trial, around 76% could be extubated without weaning. The remaining patients were randomly assigned to be weaned using 2-hour spontaneous breathing trials with various modalities: daily multiple tube T/CPAP breathing, PS mode, and SIMV. The Esteban trial also included a fourth arm, tube T trials once daily. There was no difference in ventilation duration between tube T and PS, and trends were opposite in the two studies: Esteban et al. (1995) favoured tube T weaning, while Brochard et al. (1994) favoured PS. Both studies showed shorter ventilation duration with tube T compared to SIMV. In the PS vs. SIMV comparison, both studies found trends in favour of PS, although the effect in the Brochard study was much larger.
In the paediatric population, there are few studies comparing PS to other weaning methods. Farias et al. (2001) compared spontaneous breathing trials (SBT) using PS of 10 cmH2O to a tube T trial. The rationale for using PS was to overcome endotracheal tube resistance. The 257 subjects had to tolerate the 2-hour trial (either PS or tube T) to be considered for extubation. The attending physician could interrupt SBT due to objective (e.g., increased RR or SpO2<90%) or subjective (e.g., sweating or increased respiratory effort) signs of poor tolerance. There were no differences in extubation failure rates within 48 hours (15.1% vs. 12.8%) or SBT failure (20.8% vs. 22.7%). The study concluded that a 10 cmH2O PS SBT was as effective as a tube T trial. In 2002, the same authors studied 418 patients intubated for at least 48 hours using a 2-hour SBT with tube T or 10 cmH2O PS (^60^). Of the 323 patients (77%) who passed the SBT and were extubated, 14% were re-intubated within 48 hours. Respiratory rate, tidal volume, RSBI, and maximum inspiratory negative pressure (PImáx) were poor predictors of extubation outcome. In both studies, patients underwent an SBT only when deemed ready by the attending physician, possibly not at the earliest point when an SBT could have been performed.
In adults, Esteban et al. (1997) found that two-thirds of patients passed an SBT even before weaning started. If the SBT had been performed earlier in the Farias study, there could have been an increased SBT failure rate in the tube T group compared to the PS group. Willis et al. (2005) quantified respiratory work (measured by a surrogate, the product of pressure rate) in 22 patients. They found no difference between CPAP and 5 cmH2O PS. Both provided reduced respiratory work compared to tube T (with or without heliox) or extubated patients. Patients on tube T had less respiratory work than when extubated. Takeuchi et al. (2000) demonstrated that breathing work through an ETT for infants was only marginally higher than after extubation. They also showed that 4 cmH2O PS was more than sufficient to compensate for marginal increases in respiratory work through an internal diameter of 3.5 to 4.5 mm ETT and was equivalent to breathing without the ETT. A series of studies involving 634 infants and children (Farias et al. 2001) demonstrated that a safe spontaneous breathing trial lasting up to two hours could be performed using a tube T trial for ERT. While the trend of using PS with PEEP instead of CPAP or tube T breathing to overcome ETT resistance has emerged, evidence shows that the resistance increase is minimal and the additional respiratory work insignificant. If a baby or small child cannot sustain an SBT with CPAP or a tube T for several hours, the likelihood of extubation failure is as probable as with applied PS. Additionally, PS addition likely masks respiratory failure and contributes to a higher extubation failure rate.
Myth 6: In the case of cardiopulmonary resuscitation, mechanical ventilation should not be maintained during resuscitation
Advanced cardiopulmonary resuscitation (CPR) often requires a significant number of healthcare personnel. In situations where an emergency department is overwhelmed, there is a shortage of available healthcare staff, or personnel are less trained in manual ventilation, the use of mechanical ventilation provides advantages. This allows airway-focused personnel to concentrate on other tasks during CPR, such as chest compressions, defibrillation, identifying the causes of cardiac arrest, and more (Weiss et al. 2005).
Positive pressure ventilation can be delivered through an advanced airway using a bag-valve mask (BVM) or a mechanical ventilator. It was found that both ventilation methods were equally effective in terms of arterial gas measurements in a prospective intervention study involving 122 patients with cardiac arrest (Johannigman et al. 1995).
In adults, the "six-dial strategy" has been described for mechanical ventilator programming during CPR. This involves setting six parameters: PEEP of 0 cm H2O (to favour venous return), using volumecontrolled mode with 8 ml/kg of ideal body weight and FiO2 of 100% (to ensure adequate oxygenation), respiratory rate of 10 breaths per minute (for proper ventilation), inspiratory pressure alarm set at 60 mm H2O (to deliver the tidal volume during chest compressions), trigger or sensitivity turned off (to prevent triggering during chest recoil), and an I:E ratio of 1:5 (to achieve an appropriate inspiratory time) (Sahu et al. 2020).
For children already on mechanical ventilation, the 2021 European Resuscitation Council Guidelines for Paediatric Life Support emphasise the need to ensure that the ventilator is in a volume-controlled mode, with triggers and limits deactivated. The ventilation frequency, tidal volume, and FiO2 should be appropriate for cardiopulmonary resuscitation. There is no evidence to support a specific level of PEEP during CPR. Always bear in mind that ventilator dysfunction itself could be a cause of cardiac arrest (Van de Voorde et al. 2021).
More information regarding mechanical ventilation during CPR is anticipated. Recently, a porcine model of paediatric asphyxial cardiac arrest was used to demonstrate that pressure-controlled ventilation at a rate of 20 breaths/minute with FiO2 of 100% provided adequate oxygenation and appropriate normocapnia.
Myth 7. Maintaining a SpO2 of 100% is safe and appropriate for paediatric and neonatal patients
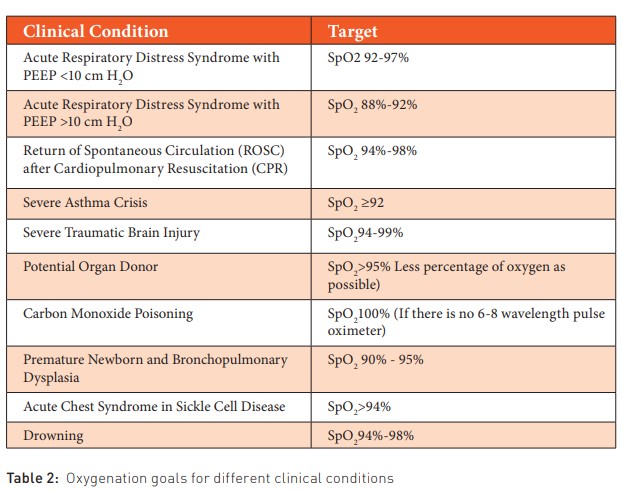
The critically ill patient presents various nuances in intensive care or emergency settings, where we must recall the oxygenation goals for each specific clinical scenario. Excessive delivery of FiO2 is linked to an excess of oxygen-free radicals. (Bohnhorst et al. 2000). We will outline the oxygenation goals for different clinical conditions, aiding in the avoidance of elevated FiO2 levels, as well as high pressures (positive end-expiratory pressure and peak inspiratory pressure) and utilised tidal volumes (Table 2).
Pulse oximeters can serve as a surrogate for arterial blood gas saturation. These devices need to be accurate across a wide range of skin tones and thicknesses and for a broad spectrum of saturations. Generally, pulse oximeters are most accurate at higher saturations, typically above 75% (Bohnhorst et al. 2000; Carter et al. 1998; Fanconi 1988).
Pulse oximetry estimates the percentage of haemoglobin saturation. It is not intended to be a substitute for blood oxygen pressure (PaO2) measurement, especially at extreme values. The relationship between PaO2 and oxygen saturation is influenced by multiple factors, including haemoglobin type and the state of the oxyhaemoglobin dissociation curve. The latter is affected by acid-base status, temperature, and 2,3-diphosphoglycerate (DPG) levels. Pulse oximetry is known to be inaccurate during periods of hypoxaemia (saturations, 85%-90%) and generally reads 98% to 100% when PaO2 exceeds 100 mm Hg. Both external and patient-related factors, including movement, ambient light, and low tissue perfusion, can interfere with its accuracy.
Pulse oximeters are not precisely calibrated for low saturations seen in cyanotic congenital heart diseases. However, there is a blue sensor designed for the saturation range found in patients with these clinical conditions. The PaO2 values need to meet the metabolic demands of a neonate range from 50 to 80 mmHg due to their high proportion of circulating foetal haemoglobin (HbF). In neonates, SpO2 levels between 85% and 95% correlate with PaO2 levels between 45 and 65 mmHg (Quine et al. 2008). However, these measurements are limited in scenarios of significant hypoaxemia or hyperoxaemia.
For saturations above 96%, PaO2 levels continue to increase without a significant change in SpO2, potentially leading to hyperoxaemia. The high affinity of HbF for oxygen shifts the haemoglobin dissociation curve to the left, resulting in relatively high saturations (85%) for PaO2 levels below the 45 mmHg threshold, leading to complications associated with hypoxaemia. This effect is more pronounced with a higher proportion of circulating HbF, and it's been known for a long time that a neonate born at 28 weeks of gestation can have a circulating HbF percentage ranging from 90% to 97%.
Maintaining high saturations in preterm neonates (96% to 99%) is associated with increased mortality, a higher risk of bronchopulmonary dysplasia (BPD), no reduction in the need for ablation of avascular peripheral retinas in neonates with retinopathy of prematurity (ROP), and longer hospitalisations (STOP-ROP 2000). Using lower saturation ranges in preterm neonates (85% to 89%) is associated with higher mortality in babies less than 36 weeks of gestation, a higher incidence of necrotising enterocolitis, a greater risk of patent ductus arteriosus persistence requiring surgical closure, a decrease in ROP incidence requiring treatment, and a lower risk of requiring supplementary oxygen at 36 weeks corrected gestational age (Askie et al. 2018). Setting the saturation target for preterm neonates between 90% and 95% is a sensible strategy to minimise risks of extreme oxygenation and avoid complications associated with lower saturation ranges (Polin and Bateman 2013) (Table 2).
Conflict of Interest
None.
References:
Askie LM, Darlow BA, Finer N et al. (2018) Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA. 319(21):2190-2201.
Bhalla AK, Klein MJ, Emeriaud G et al. (2021) Adherence to Lung-Protective Ventilation Principles in Pediatric Acute Respiratory Distress Syndrome: A Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology Study. Critical care medicine. 49(10):1779-1789.
Black AE, Hatch DJ, Nauth-Misir N (1990) Complications of nasotracheal intubation in neonates, infants and children: a review of 4 years’ experience in a children’s hospital.Br J Anaesth. 65:461–467.
Bohnhorst B, Peter CS, Poets CF (2000) Pulse oximeters’ reliability in detecting hypoxemia and bradycardia: comparison between a conventional and two new generation oximeters. Crit Care Med. 28(5):1565-1568.
Boles JM, Bion J, Connors A et al. (2007) Weaning from mechanical ventilation. Eur Respir J. 29:1033–56.
Brochard L, Rauss A, Benito S, et al. (1994) Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 150(4):896-903.
Brown MK, Dibiasi RM (2011) Mechanical ventilation of the premature neonate. Respir Care. 56:1298-311.
Carter BG, Carlin JB, Tibballs J et al. (1998) Accuracy of two pulse oximeters at low arterial hemoglobinoxygen saturation. Crit Care Med. 26(6):1128-1133.
Chambers NA, Ramgolam A, Sommerfield D et al. (2018) Cuffed vs. Uncuffed tracheal tubes in children: a randomised controlled trial comparing leak, tidal volume and complications. Anaesthesia. 73:160–168.
Chavez A, dela Cruz R, Zaritsky A (2006) Spontaneous breathing trial predicts successful extubation in infants and children. Pediatr Crit Care Med. 7(4):324–328.
Chen L, Zhang J, Pan G et al. (2018) Cuffed versus uncuffed endotracheal tubes in pediatrics: a meta-analysis. Open Med (Wars). 13:366–373.
De Orange FA, Andrade RG, Lemos A et al. (2017) Cuffed versus uncuffed endotracheal tubes for general anaesthesia in children aged eight years and under. Cochrane Database Syst Rev. 11:CD011954.
Diaz E, Lorente L, Valles J, Rello J (2010) Mechanical ventilation associated pneumonia. Med Intensiva. 34:318–24.
Esteban A, Alía I, Gordo F et al. (1997) Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 156 (2 Pt 1):459–465.
Esteban A, Alia I, Tobin MJ et al. (1999) Spanish Lung Failure Collaborative Group. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Am J Respir Crit Care Med. 159(2):512–518.
Esteban A, Frutos F, Tobin MJ et al. (1995) Spanish Lung Failure Collaborative Group. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 332:345–350.
Fanconi S (1988) Reliability of pulse oximetry in hypoxic infants. J Pediatr. 112(3):424-427.
Farias JA, Retta A, Alia I et al. (2001) A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med 27(10):1649–1654.
Farias JA, Alia I, Retta A et al. (2002) An evaluation of extubation failure predictors in mechanically ventilated infants and children. Intensive Care Med. 28(6):752–757.
Farias JA, Frutos F, Esteban A et al. (2004) What is the daily practice of mechanical ventilation in pediatric intensive care units. A multicenter study. Intensive Care Med. 30:918–25.
Farias JA, Alía I, Esteban A et al. (1998) Weaning from mechanical ventilation in pediatric intensive care patients. Intensive Care Med. 24:1070–5.
Farias JA, Monteverde E (2006) We need to predict extubation failure. J Pediatr. 82:322–4.
Greenough A, Morley CJ, Pool J (1986) Fighting the ventilator. Are fast rates an effective alternative to paralysis? Early Human Dev. 13:189-94.
Greenouch A (2001) Update on patient-triggered ventilation. Clin Perinatol. 28:533-46.
Gregory GA, Kitterman JA, Phibbs RH (1971) Treatment of the idiopathic respiratory-distress syndrome with continuous positive airway pressure. New Engl J Med. 284:1333-40.
Grupo Respiratorio y Surfactante de la Sociedad Española de Neonatología (2008) Recomendaciones para la asistencia respiratoria en el recién nacido (I). An Pediatr (Barc). 68:516-24.
Hampson NB (1998) Pulse oximetry in severe carbon monoxide poisoning. Chest. 114(4):1036-1041.
Higgins RD, Bancalari E, Willinger M, Raju TNK (2007) Executive summary of the workshops on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics. 119:790-6.
Hummler H, Schulze A (2009) New and alternative modes of mechanical ventilation in neonates. Semin Fetal Neonatal Med. 14:42-8.
Johannigman JA, Branson RD, Johnson DJ et al. (1995) Out-of-hospital ventilation: bag-valve device vs. Transport ventilator. Acad Emerg Med. 2(8):719–724.
Keszler M (2009) State of the art in conventional mechanical ventilation. J Perinatol. 29:262-75.
Kleinman ME, Chameides L, Schexnayder SM et al. (2010) Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 122(suppl 3):S876–S908.
Klingenberg C, Wheeler KI, McCallion N et al. (2017) Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev. 10:CD003666.
Kneyber MCJ, de Luca D, Calderini E et al. (2017) Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med. 43(12):1764-1780.
Kochanek PM, Tasker RC, Bell MJ et al. (2019) Management of Pediatric Severe Traumatic Brain Injury: 2019 Consensus and Guidelines-Based Algorithm for First and Second Tier Therapies. Pediatr Crit Care Med. 20(3):269-279.
Kurachek SC, Newth CJ, Quasney MW et al. (2003) Extubation failure in pediatric intensive care: A multiple-center study of risk factors and outcomes. Crit Care Med. 31:2657–64.
Lapid FM, O’Brien CE, Kudchadkar SR et al. (2020) The use of pressure controlled mechanical ventilation in a swine model of intraoperative pediatric cardiac arrest. Paediatr Anaesth. 30:462-8.
Meade M, Guyatt G, Sinuff T et al. (2001) Trials comparing alternative weaning modes and discontinuation assessments. Chest. 120 6 Suppl:425S–437S.
Medina VA (2015) Manual de ventilacion mecanica pediatrica y neonatal. 5ª Edition.
Newth CJ, Venkataraman S, Willson DF et al. (2009) Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. 10:1–11.
Orsborn J, Graham J, Moss M et al. (2016) Pediatric Endotracheal Tube Cuff Pressures During Aeromedical Transport.Pediatr Emerg Care. 32:20–22.
Perlman JM, McMenamin JB, Volpe JJ (1983) Fluctuating cerebral blood-flow velocity in respiratory distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med. 309:204-9.
Pinsky MR (2000) Breathing as exercise: The cardiovascular response to weaning from mechanical ventilation. Intensive Care Med. 26:1164–6.
Polin RA, Bateman D (2013) Oxygen-saturation targets in preterm infants. The New England Journal of Medicine. 368(22):2141-2142.
Rodrigo GJ, Rodriquez Verde M, Peregalli V, Rodrigo C (2003) Effects of short-term 28% and 100% oxygen on paco2 and peak expiratory flow rate in acute asthma: a randomized trial. Chest. 124(4):1312-7.
Sahu AK, Timilsina G, Mathew R et al. (2020) Six-dial Strategy - Mechanical Ventilation during Cardiopulmonary Resuscitation. Indian J Crit Care Med. 24(6): 487–489.
Schweiger C, Marostica PJ, Smith MM et al. (2013) Incidence of post-intubation subglottic stenosis in children: prospective study. J Laryngol Otol. 127:399–403.
Shemie SD, Ross H, Pagliarello J et al. (2006) Organ donor management in Canada: recommendations of the forum on medical management to optimize donor organ potential. Can Med Assoc J. 174:S13–S30.
Shi F, Xiao Y, Xiong W et al. (2016) Cuffed versus uncuffed endotracheal tubes in children: a meta-analysis. J Anesth. 30:3–11.
Slutsky AS (2005) Ventilator-induced lung injury: From barotrauma to biotrauma. Respir Care. 50:646–59.
Stark AR, Bascom R, Frantz ID (1979) Muscle relaxation in mechanically ventilated infants. J Pediatr. 94:439-43.
Stoller JK (1999) The history of intubation, tracheotomy and airway appliances, Respir Care. 44:595.
Takeuchi M, Imanaka H, Miyano H et al. (2000) Effect of patient-triggered ventilation on respiratory workload in infants after cardiac surgery. Anesthesiology. 93(5):1238-44.
The Pediatric Acute Lung Injury Consensus Conference Group Pediatric Acute Respiratory Distress Syndrome (2015) Pediatric Critical Care Medicine. 16(5):428-439.
Thomas R, Rao S, Minutillo C (2016) Cuffed endotracheal tubes for neonates and young infants: a comprehensive review. Archives of Disease in Childhood – Fetal and Neonatal Edition. 101:F168-F174.
Thomas R, Rao S, Erickson S et al. (2019) Cuffed versus uncuffed endotracheal tubes for ventilation of neonates: A Pilot RCT. Journal of Paediatrics and Child Health. 55(S1).
Topjian AA, Raymond TT, Atkins D et al. (2020) Part 4: pediatric basic and advanced life support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 142(suppl 2):S469–S523.
Van de Voorde P, Nigel M. Turner JD et al. (2021) European Resuscitation Council Guidelines 2021: Paediatric Life Support. Resuscitation, 161:327-387.
Weiss SJ, Ernst AA, Jones R et al. (2005) Automatic transport ventilator vs bag valve in the EMS setting: a prospective, Randomized trial. South Med J. 98(10):970–976.
Weiss M, Dullenkopf A, Fischer JE et al. (2009) European Paediatric Endotracheal Intubation Study Group. Prospective randomized controlled multicentre trial of cuffed or uncuffed endotracheal tubes in small children. Br J Anaesth. 103:867–873.
Willis BC, Graham AS, Yoon E et al. (2005) Pressure-rate products and phase angles in children on minimal support ventilation and after extubation. Intensive Care Med. 31(12):1700–1705.
Zenteno D, Torres-Puebla G, Navarro X et al. (2021) Experience in a Pediatric Prolonged Mechanical Ventilation Unit from a public hospital in Chile. Archivos argentinos de pediatria, 119(1):25-31.























