FDA Clears Siemens RT Workflow Features
RT Pro edition enhances radiation oncology workflow on company’s SOMATOM Definition AS Open CT simulator
Siemens Healthcare has announced that the US Food and Drug Administration (FDA) has cleared the RT Pro edition – a package of features designed to enhance radiation oncology workflow for Siemens’ SOMATOM Definition AS Open CT simulator.
The RT Pro edition of the SOMATOM Definition AS Open helps deliver even higher image quality for all needs in radiation oncology – including large patients, patients with metal implants, and those who experience tumor motion.
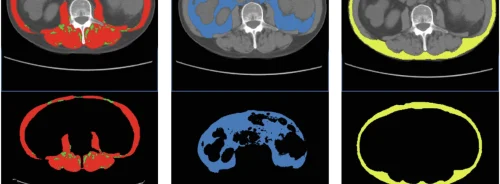
Innovations in the RT Pro edition include MARIS (Metal Artifact Reduction in Image Space), which helps ensure accurate CT data for treatment planning by reducing image-distorting beam-hardening artifacts caused by a patient’s metal implants.
Added to Siemens’ respiratory gating solution, the t-minIP and t-MIP features visualise tumor movement in one image, which is particularly useful when analysing tumor motion and contouring. Also, Siemens’ HD FoV Pro algorithm uses intelligent contour and attenuation estimation to further improve visibility outside the scan field-of-view (sFoV) through the extended 80-cm FoV, ultimately increasing image accuracy for treatment planning. MARIS, t-minIP, t-MIP, and HD FoV Pro are all standard with the RT Pro edition.
Finally, the previously available Dual Energy feature is included as an option on the RT Pro edition, helping to reduce metal artifacts and increase soft tissue contrast.
Source: Siemens
27 January 2014
Latest Articles
CT, Imaging, Siemens, Radiation, Oncology, Tumours, Implants, Visualisation, CT scans, FDA
FDA Clears Siemens RT Workflow Features RT Pro edition enhances radiation oncology workflow on company’s SOMATOM Definition AS Open CT simulator Sie...