Mitral valve prolapse (MVP) is a common valvular disease, affecting 2%–3% of the general population and generally having a good prognosis unless accompanied by significant mitral regurgitation or left ventricular (LV) dysfunction. However, some patients with MVP experience sustained ventricular tachycardia (VT) and sudden cardiac death (SCD) despite not having moderate to severe mitral regurgitation or LV dysfunction, a condition known as arrhythmic MVP. Identifying the risk factors for arrhythmic MVP is challenging. Clinical and imaging studies have suggested associations with mitral annulus disjunction (MAD), prolapse severity, and myocardial fibrosis, but the significance of these factors is still debated. Recent findings emphasize myocardial fibrosis, detectable via late gadolinium enhancement (LGE) cardiac MRI, as an important marker over MAD. The complexity of MVP's features makes it difficult to identify proarrhythmic characteristics. To address this, a recent study applied unsupervised machine learning to a comprehensive data set from an international registry of MVP patients, aiming to uncover new phenotypic features linked to arrhythmic outcomes through longitudinal analysis.
Study Design and Patient Selection
This study is based on an international, multicenter, longitudinal, retrospective registry including patients with mitral valve prolapse (MVP) without significant mitral regurgitation or left ventricular (LV) dysfunction who underwent cardiac MRI. Ethics approval was obtained from all 15 participating centers, and all patients provided written informed consent. Patients were included if they were 18 years or older, had MVP confirmed by cardiac MRI, and had clinical information and continuous ECG monitoring available within three months of the MRI. LGE imaging was also required. Exclusion criteria included the presence of cardiomyopathy, an LV ejection fraction less than 40%, ischemic or congenital heart disease, inflammatory heart disease, moderate or greater mitral regurgitation, and participation in competitive sports. Patients with LV ejection fractions between 40% and 55% were included.
MRI Protocol and Variable Selection
Cardiac MRI was performed using a 1.5-T system with specific software and equipment. Ventricular volumes, mass, and function, as well as atrial areas, were measured. MVP was defined as a displacement of the mitral valve leaflet of 2.0 mm or more into the left atrium. Mitral annulus disjunction (MAD) was defined as a separation of 2.0 mm or more between the left atrial wall/mitral valve junction and the LV inferolateral wall. LGE was considered present if visible in two orthogonal views or on the same image orientation after swapping phase-frequency direction and was quantified as the percentage of LV mass. Phenotypic clusters were created using 32 demographic, clinical, and cardiac MRI variables. History of malignant ventricular arrhythmias at baseline was excluded, as were five highly correlated variables to mitigate collinearity, leaving 27 variables for analysis. A sensitivity analysis excluded patients with malignant ventricular arrhythmias at baseline and included the baseline burden of ventricular arrhythmias.
Clustering Methodology and Statistical Analysis
Various methods were used to determine the optimal number of clusters, with the NbClust package in R providing the final determination. Hierarchical k-means clustering was used to identify phenotypic groups of patients with similar characteristics, ensuring robustness through 1000 initializations. Variables were ranked using a gradient-boosting model, and SHapley Additive exPlanations (SHAP) assessed the discriminative influence of variables for each cluster. The study end point was a composite of sustained VT, (aborted) SCD, or unexplained syncope during follow-up. Follow-up began at the date of cardiac MRI and continued until June 2020. Noncardiac deaths were censored. Two cardiologists, blinded to MRI results, adjudicated events. Statistical analyses included descriptive statistics for each cluster, incidence rates, Kaplan-Meier curves, and multivariable Cox proportional hazards analysis, performed using Stata SE version 17 and R version 4.2.1. An α level of 0.05 was used for all tests, which were two-tailed.
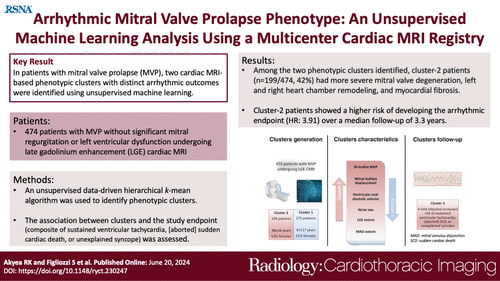
Image Credit: Radiology- Cardiothoracic Imaging
Patient Characteristics and Phenotypic Clusters
A total of 474 patients with isolated mitral valve prolapse (MVP) were included in the study, with a mean age of 47 years and a gender distribution of 51.5% female and 48.5% male. Two phenotypic clusters were identified. Cluster 2 had a higher prevalence of bileaflet prolapse, mitral annulus disjunction (MAD), greater leaflet displacement, and larger longitudinal extent of MAD compared to Cluster 1. These structural differences in Cluster 2 were associated with a higher prevalence and extent of late gadolinium enhancement (LGE) and larger biventricular and atrial dimensions. The importance of variables for predicting clusters was assessed using Shapley values, with bileaflet MVP, leaflet displacement, end-diastolic volumes, and LGE extent being the most significant predictors. During a median follow-up of 39 months, 18 patients experienced adverse events, with an overall incidence rate of 12.0 per 1000 person-years. Cluster 1 had a lower incidence rate (6.4 per 1000 person-years) compared to Cluster 2 (21.5 per 1000 person-years), yielding a hazard ratio (HR) of 5.30. After adjusting for LGE extent, Cluster 2 patients still had a significantly higher risk of adverse events (HR: 3.79). A sensitivity analysis with 456 patients confirmed the presence of two phenotypic clusters and showed that ventricular ectopic beats and nonsustained ventricular tachycardia had a negligible contribution to cluster identification. Adjusting for LGE extent, Cluster 2 patients had a higher likelihood of experiencing adverse events (HR: 4.03).
Complexity in Predicting MVP Outcomes
The study highlights the complexity of predicting adverse outcomes in patients with mitral valve prolapse (MVP) who do not exhibit significant left ventricular (LV) dysfunction or mitral regurgitation. It acknowledges that while LV dysfunction and severe mitral regurgitation are recognized risk factors, most sudden cardiac deaths (SCD) in MVP patients occur without these conditions. Using machine learning on data from 474 patients, the study identified two phenotypic clusters based on demographic, clinical, and cardiac MRI features. Cluster 2 patients exhibited more severe MVP characteristics, such as higher bileaflet prolapse, more pronounced leaflet displacement, larger heart chambers, and greater myocardial fibrosis, leading to a fourfold increase in the likelihood of SCD, sustained ventricular tachycardia (VT), or unexplained syncope over a median follow-up of more than three years. Cluster 1 patients, in contrast, had a lower incidence of these adverse outcomes.
Prognostic Implications and Structural Changes
The study suggests that isolated MVP does not generally predict poor prognosis unless associated with significant structural changes, such as myocardial fibrosis and chamber dilatation. Despite the prevalence of myocardial fibrosis being high, its presence alone did not predict adverse outcomes. The study calls for a more integrated approach combining multiple morphofunctional parameters for better risk stratification. Mitral annulus disjunction (MAD) did not significantly contribute to cluster differentiation, which contrasts with earlier studies suggesting its prognostic value. Differences in study samples and definitions of MAD might explain this discrepancy. The extent of MAD was more significant in cluster 2, supporting its association with arrhythmias due to mechanical stress and subsequent electrophysiological changes. Larger ventricles and atria in cluster 2 patients suggest a complex interplay between genetic factors and the “third chamber” effect of prolapsing mitral leaflets. Advanced cardiac MRI-based features were more predictive of outcomes than demographic or clinical features, emphasizing the need for high-resolution imaging in MVP assessment.
The study's limitations include potential selection bias, the inability to incorporate all imaging parameters or ECG features due to data constraints, and the need for further research to confirm these findings. Nevertheless, the study advocates for the use of advanced cardiovascular imaging to improve arrhythmic risk stratification in MVP patients without significant mitral regurgitation or LV dysfunction.
Source: Radiology- Cardiothoracic Imaging
Image Credit: iStock