Using the traditional compartmentalised approach, numerous tests may be ordered, including a CT of the chest with contrast, ultrasound studies of the abdomen and lower extremities, and an echocardiogram. This approach leads to the inherent clinical and time dissociation implicit with consultative radiology and echocardiography. Alternatively, a critical care physician may perform a point-of-care ultrasound (POCUS) exam at the bedside, integrating the results to establish a diagnosis and guide patient management.
Physicians have used ultrasonography for more than half a century to aid diagnosis and guide procedures. With technological advancement, ultrasound equipment became more compact and led to the advent of POCUS, which is defined as ultrasonography brought to the patient and performed by the provider in real time (Moore and Copel 2011). The realisation that physicians can see and assess physiological function in real time has been a tipping point in critical care medicine and has revolutionised the care of patients in the intensive care unit (ICU). This dynamic imaging is a complete paradigm shift from the traditional consultative approach where technologists and physicians not intimately involved in patient care are responsible for image acquisition and interpretation. This eliminates clinical and time dissociation in patient care, allows a timely diagnosis without potentially dangerous patient transport and reduces radiation exposure (Moore and Copel 2011). The critical care literature is replete with studies supporting the use of POCUS, and as an increasing number of providers acquire critical care ultrasonography training, POCUS is becoming the standard of care in the ICU (Moore and Copel 2011; Killu et al. 2014).
Thoracic ultrasonography
While the differential diagnosis of respiratory failure may be broad, thoracic ultrasound allows the intensivist to characterise the abnormality by determining the predominant pathophysiologic process present. Thoracic ultrasound may determine if the patient has predominately wet lungs (cardiogenic or non-cardiogenic pulmonary oedema), an alveolar consolidation pattern (acute respiratory distress syndrome [ARDS], pneumonia, or atelectasis), a pneumothorax, a pleural effusion, or any combination. The accuracy, sensitivity, and specificity of thoracic ultrasound are similar to chest CT in patients who present with respiratory failure. Pioneering work by the intensivist
Daniel Lichtenstein led to the development of the basic lung ultrasound findings used in the ICU.
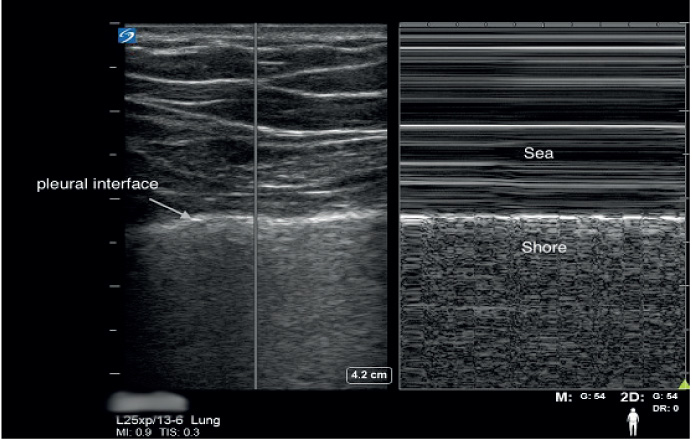
Lung sliding with an A line pattern indicates that the visceral pleural surface is moving freely with respiration against the parietal pleura, and defines a normal aeration pattern. When observed in multiple places on the thorax this indicates that major pleural or parenchymal abnormality is unlikely to exist. Lung sliding also rules out a pneumothorax with a negative predictive value of 100% at the site of the ultrasound probe (Lichtenstein and Menu 1995). A lung point is the point where normal pleural interface contacts the boundary of the pneumothorax and when observed is 100% specific for a pneumothorax (Lichtenstein et al. 2005). A recent meta-analysis concluded that ultrasound was superior to chest radiography in detecting a pneumothorax (Arajhi et al. 2012). Trained providers can easily use POCUS for immediate diagnosis of this condition instead of waiting for a formal radiograph.
Thoracic ultrasonography may help in identification of the cause of respiratory failure. A-lines are horizontal repetitions of the pleural line, and indicate a normal aeration pattern of the lung. However, when the sub pleural interlobular septa are oedematous, hyperechoic comet tail artifacts called B-lines arise, which indicate the presence of an alveolar interstitial syndrome. When bilateral, multiple B-lines can signify cardiogenic or non-cardiogenic pulmonary oedema; when unilateral they likely represent infection, or rarely unilateral pulmonary oedema (re-expansion oedema or mitral regurgitation) (Lichtenstein and Mezière 2008). Since B-lines are dynamic and disappear as the underlying process improves, repeat ultrasound exams can be used to monitor a patient’s response to treatment. A-line versus B-line patterns on lung ultrasonography also allow the differentiation of exacerbations of chronic obstructive pulmonary disease (COPD) and pulmonary oedema secondary to decompensated heart failure (Lichtenstein and Mezière 1998). Thoracic ultrasonography also has 90% sensitivity and 98% specificity in detecting alveolar consolidations, which have a sonographic appearance similar to that of the liver, a pattern referred to as hepatisation of the lung. Ultrasound is arguably the best radiographic modality for the diagnosis and characterisation of a pleural effusion, and allows safe and accurate placement of various chest tubes if clinically indicated. Diaphragmatic function is also accurately assessed with ultrasound and may help in deciding when to liberate patients from mechanical ventilation (Ferrari et al. 2014). In the BLUE-protocol described by Lichtenstein, profiles have been designed to allow rapid diagnosis of the common causes of acute respiratory failure with an accuracy > 90% (Lichtenstein and Mezière 1998).
Critical care echocardiography
The categorisation of shock state with goal-directed echocardiography has logical benefit for patients in cardiorespiratory failure. Using limited echocardiographic views, the intensivist can rapidly determine whether the shock state is distributive, obstructive (massive pulmonary embolus or tamponade), cardiogenic or hypovolaemic. Life-threatening processes are identified immediately (massive pericardial effusion) and allow coordination of emergent procedures (pericardiocentesis or thrombolysis) Key findings, such as right ventricular pressure overload from heart-mechanical ventilator interactions, or preload sensitivity in undifferentiated shock, is readily identified by the intensivist with basic goal-directed echocardiography.
Echocardiography also allows rapid assessment of the causes of respiratory failure that derive from cardiac dysfunction. Goal-directed echocardiogram performed during cardiopulmonary resuscitation may identify a large pericardial effusion, a flail mitral leaflet, an intra-cavitary thrombus, or a dilated hypokinetic right ventricle (RV) with apical sparing (McConnell’s sign). These findings may guide the team in performing emergency interventions. Echocardiography may also identify patients where continued cardiopulmonary resuscitation will not be successful (Varriale and Maldonado 1997; Blaivas and Fox 2001).
Echocardiography also allows the intensivist to monitor the evolution of disease, observe the response to potentially therapeutic interventions, such as inotropic drugs, and to search for new problems that arise during the course of a critical illness.
Goal-directed vascular ultrasonography
As an ever-threatening nemesis to the intensivist, the overt or covert presence of deep vein thrombosis (DVT) and pulmonary embolism are a frequent cause of morbidity and mortality in the ICU. Fortunately, numerous studies have confirmed that non-radiologists can become competent at diagnosing a DVT with an accuracy comparable to radiologists, and only compression ultrasonography is required to make a diagnosis of a DVT (colour and spectral Doppler are unnecessary) (Lensing et al. 1997).
The ultrasonographic appearance of a typical thrombus is a vein with an intraluminal echogenic focus with non-compressible walls. However, fresh, immature thrombi may not be echogenic, and therefore the primary diagnostic criterion is a lack of vessel compressibility and not visualisation of the thrombus.
A limited compression ultrasound study, with five compression points, has a sensitivity and specificity similar to that of a complete examination in detecting a lower extremity DVT (Lensing et al. 1989; Crisp et al. 2010), and is particularly useful when combined with goal-directed lung and cardiac ultrasound in a haemodynamically unstable patient.
Abdominal goal-directed exams
While the abdominal space is complex and the mastery of abdominal ultrasonography is time-consuming, goal-directed abdominal ultrasound is easy to perform and has numerous applications in the ICU. These include the detection of ascites, assessment for hydronephrosis, distension of the bladder, assessment of the aorta and gross organ abnormalities.
Abdominal ultrasound is an optimal first-line imaging modality for the evaluation of peritoneal free fluid. The “focused assessment with sonography for trauma” (FAST) protocol is used in evaluating the patient for free peritoneal fluid from haemorrhage (American Institute of Ultrasound in Medicine 2014). Ultrasound has been shown to be very sensitive and specific for haemoperitoneum, but can be used for evaluation of free peritoneal fluid from any cause.
While the hepatobiliary and pelvic structures are amenable to imaging with bedside ultrasound, the intensivist must use caution in making diagnoses out of their comfort zone, as there are numerous normal variants that may be confused for pathology. A focused US of the kidney and the bladder should be incorporated into the exam for any patient presenting with renal failure, haematuria, urinary retention, abdominal/flank pain or renal trauma. Goal-directed abdominal ultrasound can be used to detect hydronephrosis and urinary obstruction as a cause of acute renal failure. Hydronephrosis is diagnosed when an anechoic area is noted within the renal sinus, which is not vascular in origin (Wang and Chen 2007).
The intensivist can also use ultrasonography in patients with acute abdominal conditions to rule out free air, observe peristalsis, and assess the abdominal aorta to detect abdominal aortic aneurysm rupture or abdominal aortic dissection.
Whole-body ultrasonography?
The benefit of goal-directed ultrasonography is that it relies on the clinician. Since POCUS is used as an extension of the physical exam it is naturally applicable in a ‘head-to-toe’ fashion to augment the diagnostic process. However, this is not practical, just as a daily fundoscopic exam in a patient with status asthmaticus seems like a waste of time. We perform goal-directed ultrasound as the name implies, where it is needed. All patients in respiratory failure have a thoracic ultrasound, DVT study, and echocardiography. A negative DVT study in a patient with ARDS allows for a baseline to compare should the clinical situation change, as regularly occurs with critically ill patients. Rapid changes in cardiac contractility are seen frequently with normal to hyperdynamic left ventricular (LV) function reduced to either severe global dysfunction or with increasing frequency apical ballooning disease. Patients in renal failure have abdominal ultrasonography to rule out hydronephrosis and to evaluate the bladder; and so on.
Conclusion
We do not deny the life-saving contribution of medical advancement. However, evidence-based medicine through controlled clinical trials has not confirmed the benefit of mechanical ventilators, CAT scans or routine blood analysis. Yet we hold these truths to be self-evident and all intensivists must have intimate knowledge of these tools. POCUS should be no different; a tool used by all intensivists because the logic and evidence of its utility is overwhelming and self-evident. Excuses, such as “I do not need it”, “How can I learn it?”, “It is too time consuming” etc., have all been overcome by thousands of intensivists worldwide through successful training programmes and mentorship. Old dogs can learn new tricks! As illustrated by this case, POCUS is an extremely valuable tool, which should be used by all intensivists in the evaluation of critically ill patients. It is now difficult to find a critical care journal that does not have articles supporting the use of POCUS and intensivists who use it regularly feel as though they could never go back to practising without it. POCUS signals an era of time-saving and cost-efficient “visual” medicine and once you start using ultrasonography there is definitely no going back.
Returning to our original caseWe met our patient in the
emergency department; a chest CT with contrast was ordered to rule out a
pulmonary embolus, an abdominal CT was ordered to rule out
hydronephrosis, and an echocardiogram was ordered to rule out acute cor
pulmonale and a pregnancy-related cardiomyopathy. The patient was
intubated, hypotensive and severely hypoxaemic. What did we do?
Time 0: A thoracic ultrasound revealed bilateral B-lines
consistent with pulmonary oedema of unclear aetiology: no alveolar
consolidation or pleural effusion was present.
Time 1.5 minutes: Goal-directed
echocardiography revealed normal LV function, normal RV size and
function, no pericardial effusion, grossly normal valvular function, and
a large inferior vena cava without respiratory variation.
Time 2.5-5 minutes: A goal-directed abdominal ultrasound did not reveal significant hydronephrosis and DVT compression ultrasound was normal.
Time 5 minutes: Based
on the lab results and findings of the POCUS exam, it was determined
that the patient had preeclampsia with ARDS. This diagnosis was rendered
in just 5 minutes by an intensivist with basic training in critical
care ultrasonography, saving this very unstable patient from a dangerous
delay in diagnosis as well as unnecessary transport and radiation exposure.
|
Conflict of interest
Gulrukh Zaidi declares that she has no conflict of interest. Seth Koenig declares that he has no conflict of interest.