ICU Management & Practice, Volume 18 - Issue 1, 2018
Hypothermia (HT) is a cornerstone of neuroprotective strategies and has been used in critical care for acutely brain injured adult patients for many years. This review aims to discuss the clinical evidence supporting the use of HT in neurocritical care patients beyond care after cardiac arrest (CA), such as traumatic brain injury (TBI), acute ischaemic stroke (AIS), non-traumatic intracerebral haemorrhage (ICH) and subarachnoid haemorrhage (SAH). Despite promising results in small clinical trials and laboratory studies, HT did not improve outcome in large multicentre trials. Similarly, the use of HT as a specific intervention to treat secondary brain damage failed to improve outcome in humans. Despite these negative results, targeted temperature management (TTM) remains a crucial intervention in neurocritical care as part of the emerging principle of individualised medicine. Based on the pathophysiology of harmful effects associated with fever, the concept of normothermia has been implemented in most neurointensive care units to prevent periods of fever. In the following we will review the current status of HT as well as ongoing clinical trials of TTM (see summary in Table) and provide some thoughts for designing future studies.
From a conceptual point of view, mild HT (32–34°C) can be used as prophylactic intervention early after acute brain injury (ABI) or as a symptom-based treatment for e.g. refractory intracranial hypertension, brain oedema, vasospasm after SAH and other complications. If HT is used as specific therapy it is important to first prove that the resolution of such complications is in fact associated with improved functional outcomes before an intervention like HT with potential detrimental side effects is implemented. Though experimental data suggest a significant neuroprotective effect for TTM, large multicentre clinical trials investigating HT in ABI failed to demonstrate an unambiguous clinical benefit.
You might also like: Therapeutic Hypothermia: Time for a Moratorium
Hypothermia as early prophylactic neuroprotective treatment
Traumatic brain injury
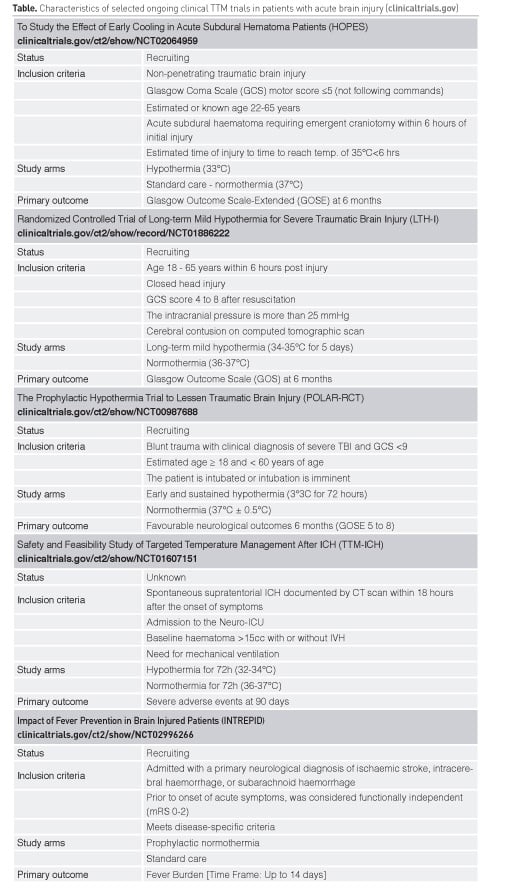
TBI is a dynamic disease with distinct pathophysiologic mechanisms depending on the injury type, injury severity and the time elapsed since the initial trauma (Maas et al. 2017). Secondary injury mechanisms including excitotoxicity, neuro-inflammation, ionic fluctuations, necrosis and apoptotic cell death have been well characterised in preclinical studies. Most of these factors are sensitive to temperature changes and may be aggravated by intracranial or extracranial insults. HT has been used in the treatment of TBI for many years and can effectively ameliorate secondary injury. Despite promising results from experimental studies and small clinical trials (Polderman 2008), large multicentre trials failed to translate the putative benefits of HT into improved outcome in TBI patients (Clifton et al. 2001; 2011; Maekawa et al. 2015). A trend towards improved functional recovery was seen in TBI patients who were hypothermic on admission, suggesting that ultra-early initiation of HT may be important (Clifton et al. 2001). In the National Acute Brain Injury study: Hypothermia II (NABISH II) trial mean time to 35°C was reached after 2.6h and time to 33° was 4.4h (Clifton et al. 2011). However, enrolment was stopped for futility when interim analyses found no difference in mortality or neurological outcomes. Post-hoc analysis suggested a benefit for severe TBI patients with focal lesions undergoing haematoma removal, pointing to the pathophysiologic concept of reperfusion injury that may be ameliorated by HT (Clifton et al. 2012). The Hypothermia for Patients requiring Evacuation of Subdural Hematoma (HOPES) trial is currently investigating this hypothesis in TBI patients requiring emergent craniotomy (clinicaltrials.gov/ct2/show/NCT02064959). Based on the concept of ongoing secondary injury beyond 48h after severe TBI, long-term HT (4-6 d) was associated with significantly higher rate of favourable outcomes at 6 months when compared to short-term HT (1-3 d) in a single-centre trial including 215 severe TBI patients with cerebral contusion and intracranial hypertension (Jiang et al. 2006). Extended cooling over 5 days is currently explored in the Long-term Mild Hypothermia for Severe Traumatic Brain Injury (LTH-I) trial (clinicaltrials.gov/ct2/show/NCT01886222; Lei et al. 2015). In addition, the POLAR-RCT (Prophylactic Hypothermia Trial to Lessen Traumatic Brain Injury) trial aims to investigate the effect of early (prehospital) and sustained (72 h) mild HT on 6 months neurological outcomes in severe TBI patients, and titrates rewarming speed to intracranial pressure (ICP) and mean arterial pressure (MAP) (clinicaltrials.gov/ct2/show/NCT00987688); (Nichol et al. 2015). Current Brain Trauma Foundation guidelines recommend against the use of early (within 2.5h), short-term (48h post-injury) prophylactic hypothermia in severe TBI patients with diffuse injury (Level IIB; braintrauma.org). Despite a large number of studies, there remains no high-quality evidence that prophylactic hypothermia is beneficial in the treatment of patients with severe TBI (Lewis et al. 2017).
Acute ischaemic stroke
In AIS mild HT is not effective to salvage neural tissue that has progressed irreversibly to infarction. HT can be directed at minimising the extent of secondary injury in the acute or subacute period of AIS. Based on animal data HT has a huge potential as a neuroprotective strategy when used early (within 2-3h), and mitigates ischaemic and reperfusion damage even when initiated up to 6 hours after ictus. However, clinical studies are limited by low patient numbers, slow recruitment, methodological issues and the occurrence of complications such as pneumonia and shivering, especially during HT in awake patients. So far, unequivocal efficacy of HT (33–35°C) has not yet been shown in AIS patients (Lyden et al. 2016; Piironen et al. 2014; De Georgia et al. 2004; Hemmen et al. 2010; Ovesen et al. 2013; Bi et al. 2011; Els et al. 2006), and the use outside clinical studies can currently not be recommended (Jauch et al. 2013; Ntaios et al. 2015).
The Cooling Plus Best Medical Treatment Versus Best Medical Treatment Alone for Acute Ischaemic Stroke (EuroHYP-1) trial explores the value of HT (34–35°C for 24h) within 150 minutes after start of alteplase administration and within 6 hours after stroke onset (van der Worp et al. 2014). The primary outcome is the degree of disability at 3 months measured by ordinal regression analysis of the modified Rankin Scale. This trial aims to recruit 800 patients and has recently reported safety based on preliminary analysis of 62 patients.
Intracerebral haemorrhage
Despite evidence from animal studies that HT attenuates perihaematoma oedema, inflammation and thrombin-induced injury and preserves blood-brain barrier integrity in ICH, we lack human data demonstrating improved neurologic outcome secondary to these beneficial effects of HT (Fischer et al. 2017). Two observational studies showed that mild prolonged HT (35°C over 8–10 d) had a favourable impact on perihaematomal oedema and ICP. However, no benefit for neurologic outcome was observed (Kollmar et al. 2010; Staykov et al. 2013). Although slow recruiting, the CINCH trial investigates HT at 35°C for 8 days in patients with primary ICH and large haematoma volume (25–64 mL) (Kollmar et al. 2012). A phase II prospective trial investigating 72h of mild HT (32–34°C) compared to normothermia (36–37°C) aims to include 50 supratentorial ICH patients (volume >15 ml) (clinicaltrials.gov/ct2/show/NCT01607151). For now, prophylactic mild HT is considered investigational in ICH patients and should only be applied in clinical trials (Hemphill et al. 2015). There is a strong need for prospective randomised-controlled trials further investigating the effect of hypothermia on perihaematoma oedema progression and neurological outcome.
Subarachnoid haemorrhage
Hypothermia has been used already in the 1950s as a neuroprotective strategy during aneurysm surgery in SAH patients (Botterell et al. 1956). The IHAST trial investigated HT (33°C) compared to normothermia in 1001 good-grade SAH patients during aneurysm surgery and failed to demonstrate an outcome benefit (Todd et al. 2005). Based on the reported non-significant increase in recovery, a recent Cochrane review, summarising 3 studies with 1158 participants, argued that it remains possible that intraoperative HT may be beneficial in good-grade SAH patients (Li et al. 2016). No conclusions can be drawn for poor-grade SAH patients.
Prophylactic HT was mostly tested in poor-grade SAH patients in single-centre studies underpowered to detect a difference in functional outcome (Choi et al. 2017; Gasser et al. 2003; Seule et al. 2009). An observational matched controlled study including 36 poor-grade SAH patients investigated early (<48h after ictus), mild (35°C), and prolonged (7±1 days) HT, and found a decreased rate of macro-vascular vasospasm and delayed cerebral ischaemia (DCI) (Kuramatsu et al. 2015). In a recent systematic review and meta-analysis including 9 studies the authors found a significant reduction in DCI favouring HT without an overall effect on mortality and morbidity (Yao et al. 2018). Further trials powered to detect differences in clinical outcomes are needed to investigate the potential role of HT after SAH in greater detail.
Hypothermia as symptom-based intervention (to treat raised ICP)
HT was beneficial in controlling raised ICP and improving outcome in single-centre studies; however, large multicentre trials including the recently published Eurotherm3235 trial failed to confirm these findings for a heterogeneous TBI patient population (Andrews et al. 2015). This trial examined the effect of moderate HT (32–35°C) on ICP and neurological outcome and found that titrated HT successfully reduced ICP but did not improve functional outcome at 6 months. Interestingly, there were fewer occurrences of failure of stage 2 interventions to control ICP in the HT group, and stage 3 interventions (i.e. barbiturate coma but not decompressive craniectomy) were more often used in the control group. These data support the effectiveness of HT in reducing raised ICP. The recently published French guidelines recommend considering TTM at 34–35°C to lower ICP in TBI patients with refractory intracranial hypertension (Grade 2+) (Cariou et al. 2017). Based on expert consensus, TTM at 35-37°C may also be considered in patients with ICH and SAH to lower ICP (Cariou et al. 2017).
Adverse effects associated with hypothermia
Moderate HT is a reasonably well tolerated intervention when patients are meticulously managed in an intensive care environment by experienced clinicians. Vigilant monitoring for laboratory abnormalities, cardiac monitoring and standard critical care guidelines for monitoring of infections are recommended (Madden et al. 2017). Shivering is a physiologic thermoregulatory response and is related to increased metabolism, oxygen consumption, and increased energy expenditure, which could nullify the neuroprotective benefits of TTM. Therefore routine assessment (Badjatia et al. 2008) and aggressive treatment is recommended (Choi et al. 2011; Madden et al. 2017). Another important issue in the use of therapeutic HT is the rewarming phase. It has been shown that rapid rewarming is associated with worse outcome and rebound ICP increase (Schwab et al. 2001; Jiang et al. 2006). In order to avoid this detrimental complication patients with ABI should be rewarmed to normothermia as slowly as indicated on a case-to-case basis (Polderman and Andrews 2011).
Normothermia
Acutely brain-injured patients commonly demonstrate periods of fever during the first few days after admission. Post-injury fever is associated with longer ICU stays and worse outcomes through aggravation of secondary brain injury mechanisms including excitotoxicity, increased oxygen consumption, pro-inflammatory response and increased vascular permeability leading to oedema formation with elevated ICP, increased ischaemic injury and cerebral vasospasm (Kilpatrick et al. 2000). Badjatia et al. reported a reduction in poor neurologic 12 months outcome after SAH in a case control study comparing aggressive temperature control to normothermia and conventional management of fever (Badjatia et al. 2010). Post-hoc analysis of the Japanese Brain Hypothermia (B-HYPO) study suggested a survival benefit of fever control in severe but not critical trauma patients pointing to the concept of normothermia, i.e. avoiding fever in TBI patients (Hifumi et al. 2016). Prophylactic interventions targeting normothermia have been shown to decrease the fever burden, and have been proven safe in haemorrhagic and ischaemic stroke patients (Broessner et al. 2009), and are currently under investigation in a prospective trial targeting 1176 patients (INTREPID trial, clinicaltrials.gov/ct2/show/NCT02996266). The goal of normothermia, avoiding fever, and aggressively treating fever has been suggested based on a systematic review in TBI patients and is recommended by a French expert panel reviewing the evidence for TTM in neurocritical care patients using the GRADE method (Madden and DeVon 2015; Cariou et al. 2017). TTM is therefore implemented in most neuro-intensive care units including AIS and TBI to prevent periods of fever (Rincon et al. 2014).
Summary and future perspective
The failure to translate the beneficial effect of HT observed in experimental studies to patients with ABI may reflect a limited knowledge of the multiple pathophysiologic mechanisms of secondary injury in the diverse brain pathologies. In this respect, multimodal neuromonitoring, although still mostly invasive, may help to provide insight into the pathophysiologic consequences of these conditions (Schiefecker et al. 2015). For example, increased brain temperature has recently been associated with cortical spreading depolarisations (CSD) in patients with TBI and spontaneous ICH (Schiefecker et al. 2017; Hartings et al. 2009). This is of interest because it is well known that the occurrence of CSD is extremely energy demanding and contributes to cortical lesion development following ABI (Hartings et al. 2017). This is further corroborated by the observation that clustered CSD were associated with metabolic distress and oedema development in patients with large ICH (Helbok et al. 2017). Considering the notable progress achieved by incorporating such a multimodal neuromonitoring system, this may open up the opportunity for providing individualised treatment and precision medicine in severely brain-injured patients in the future.
Conclusion
Based on the current evidence the use of prophylactic HT is not recommended in ABI patients (not considering patients with cardiac arrest) and should only be applied in the setting of clinical trials. There is a role for HT as a symptom-based intervention to e.g. treat refractory intracranial hypertension. Further research is needed to characterise the magnitude and duration of temperature modulation after ABI required for improvement of neurologic outcome. Prevention of fever and aggressive fever treatment (i.e. concept of normothermia) is feasible and recommended by experts.
Conflict of interest
Raimund Helbok received speaker 's honoraria of BARD Medical and ZOLL Medical and serves in the advisory board of the Intrepid trial (Bard Medical). Ronny Beer declares no conflict of interests.
Abbreviations
ABI acute brain injuryAIS acute ischaemic stroke
CA cardiac arrest
CSD cortical spreading depolarisations
HT hypothermia
ICH intercerebral haemorrhage
IVH intraventricular haemorrhage
SAH subarachnoid haemorrhage
TBI traumatic brain injury
TTM targeted temperature management
References:
Badjatia N, Fernandez L, Schmidt JM et al. (2010) Impact of induced normothermia on outcome after subarachnoid hemorrhage: a case-control study. Neurosurgery, 66: 696-700; Discussion 700-1.
Badjatia N, Strongilis E, Gordon E et al. (2008) Metabolic impact of shivering during therapeutic temperature modulation: the bedside shivering assessment scale. Stroke, 39, 3242-7.
Bi M, Ma Q, Zhang S et al. (2011) Local mild hypothermia with thrombolysis for acute ischemic stroke within a 6-h window. Clin Neurol Neurosurg, 11: 768-73.
Botterell EH, Lougheed WM, Scott JW et al. (1956) Hypothermia, and interruption of carotid, or carotid and vertebral circulation, in the surgical management of intracranial aneurysms. J Neurosurg, 13: 1-42.
Broessner G, Beer R, Lackner P et al. (2009) Prophylactic, endovascularly based, long-term normothermia in icu patients with severe cerebrovascular disease: bicenter prospective, randomized trial. Stroke, 40:E657-65.
Cariou A, Payen JF, Asehnoune K et al. (2017) Targeted temperature management In The ICU: guidelines from a French expert panel. Ann Intensive Care, 7: 70.
Choi HA, Ko SB, Presciutti M et al. (2011_ Prevention of shivering during therapeutic temperature modulation: the columbia anti-shivering protocol. Neurocrit Care, 14: 389-94.
Choi W, Kwon SC, Lee WJ et al.(2017) Feasibility and safety of mild therapeutic hypothermia in poor-grade subarachnoid hemorrhage: prospective pilot study. J Korean Med Sci, 32: 1337-44.
Clifton GL, Coffey CS, Fourwinds S et al. (2012) Early induction of hypothermia for evacuated intracranial hematomas: a post hoc analysis of two clinical trials. J Neurosurg, 117: 714-20.
Clifton GL, Miller ER, Choi SC et al. (2001) Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med, 344: 556-63.
Clifton GL, Valadka A, Zygun D et al. (2011) Very early hypothermia induction in patients with severe brain injury (The National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol, 10: 131-9.
De Georgia MA, Krieger DW, Abou-Chebl A et al. (2004) Cooling for acute ischemic brain damage (COOL AID): a feasibility trial of endovascular cooling. Neurology, 63: 312-7.
Els T, Oehm E, Voigt S et al. (2006) Safety and therapeutical benefit of hemicraniectomy combined with mild hypothermia in comparison with hemicraniectomy alone in patients with malignant ischemic stroke. Cerebrovasc Dis, 21: 79-85.
Fischer M, Schiefecker A, Lackner P et al. (2017) Targeted temperature management in spontaneous intracerebral hemorrhage: a systematic review. Curr Drug Targets, 18: 1430-1440.
Gasser, S, Khan, N, Yonekawa et al. (2003) Long-term hypothermia in patients with severe brain edema after poor-grade subarachnoid hemorrhage: feasibility and intensive care complications. J Neurosurg Anesthesiol, 15: 240-8.
Hartings JA, Shuttleworth CW, Kirov SA et al. (2017) The continuum of spreading depolarizations in acute cortical lesion development: examining leao's legacy. J Cereb Blood Flow Metab, 37: 1571-94.
Hartings JA, Strong AJ, Fabricius M et al. (2009) Spreading depolarizations and late secondary insults after traumatic brain injury. J Neurotrauma, 26: 1857-66.
Helbok R, Schiefecker AJ, Friberg C et al. (2017) Spreading depolarizations in patients with spontaneous intracerebral hemorrhage: association with perihematomal edema progression. J Cereb Blood Flow Metab, 37: 1871-82.
Hemmen TM, Raman R, Guluma KZ et al. (2010) Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ictus-l): final results. Stroke, 41: 2265-70.
Hemphill JC 3rd, Greenberg SM, Anderson CS et al. (2015) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 46: 2032-60.
Hifumi T, Kuroda Y, Kawakita K et al. (2016) Fever control management is preferable to mild therapeutic hypothermia in traumatic brain injury patients with abbreviated injury scale 3-4: a multi-center, randomized controlled trial. J Neurotrauma, 33: 1047-53.
Jauch EC, Saver JL, Adams HP et al. (2013) Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 44: 870-947.
Jiang JY, Xu W, Li WP et al. (2006) Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab, 26: 771-6.
Kilpatrick MM, Lowry DW, Firlik AD et al. (2000) Hyperthermia in the neurosurgical intensive care unit. Neurosurgery, 47: 850-5.
Kollmar R, Juettler E, Huttner HB et al. (2012) Cooling in intracerebral hemorrhage (cinch) trial: protocol of a randomized German-Austrian clinical trial. Int J Stroke, 7: 168-72.
Kollmar R, Staykov D, Dorfler A et al. (2010) Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke, 41: 1684-9.
Kuramatsu JB, Kollmar R, Gerner ST et al. (2015) Is hypothermia helpful in severe subarachnoid hemorrhage? an exploratory study on macro vascular spasm, delayed cerebral infarction and functional outcome after prolonged hypothermia. Cerebrovasc Dis, 40: 228-35.
Lei J, Gao G, Mao Q et al. (2015) Rationale, methodology, and implementation of a nationwide multicenter randomized controlled trial of long-term mild hypothermia for severe traumatic brain injury (the LTH-1 trial). Contemp Clin Trials, 40: 9-14.
Lewis SR, Evans DJ, Butler AR et al. (2017) Hypothermia for traumatic brain injury. Cochrane Database Syst Rev, 9: CD001048.
Li LR, You C, Chaudhary B (2016) Intraoperative mild hypothermia for postoperative neurological deficits in people with intracranial aneurysm. Cochrane Database Syst Rev, 3: CD008445.
Lyden P, Hemmen T, Grotta J et al. (2016) Results of the ICTuS 2 Trial (Intravascular Cooling in the Treatment of Stroke 2). Stroke, 47: 2888-95.
Maas AIR, Menon DK, Adelson PD et al. (2017) Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol, 16: 987-1048.
Madden LK, Devon HA (2015) A systematic review of the effects of body temperature on outcome after adult traumatic brain injury. J Neurosci Nurs, 47: 190-203.
Madden LK, Hill M, May TL et al. (2017) The implementation of targeted temperature management: an evidence-based guideline from the Neurocritical Care Society. Neurocrit Care, 27: 468-87.
Maekawa T, Yamashita S, Nagao S et al. (2015) Prolonged mild therapeutic hypothermia versus fever control with tight hemodynamic monitoring and slow rewarming in patients with severe traumatic brain injury: a randomized controlled trial. J Neurotrauma, 32: 422-9.
Nichol A, Gantner D, Presneill J et al. (2015) Protocol for a multicentre randomised controlled trial of early and sustained prophylactic hypothermia in the management of traumatic brain injury. Crit Care Resusc, 17: 92-100.
Ntaios G, Dziedzic T, Michel P et al. (2015) European Stroke Organisation (ESO) guidelines for the management of temperature in patients with acute ischemic stroke. Int J Stroke, 10: 941-9.
Ovesen C, Brizzi M, Pott FC et al. (2013) Feasibility of endovascular and surface cooling strategies in acute stroke. Acta Neurol Scand, 127: 399-405.
Piironen K, Tiainen M, Mustanoja S et al. (2014) Mild hypothermia after intravenous thrombolysis in patients with acute stroke: a randomized controlled trial. Stroke, 45: 486-91.
Polderman KH (2008) Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet, 371: 1955-69.
Polderman KH, Andrews PJ (2011) Hypothermia in patients with brain injury: the way forward? Lancet Neurol, 10: 404-5.
Rincon F, Friedman DP, Bell R et al. (2014) Targeted temperature management after intracerebral hemorrhage (TTM-ICH): methodology of a prospective randomized clinical trial. Int J Stroke, 9(5): 646-51.
Schiefecker AJ, Beer R, Broessner G et al. (2015) Can therapeutic hypothermia be guided by advanced neuromonitoring in neurocritical care patients? A review. Ther Hypothermia Temp Manag, 5: 126-34.
Schiefecker AJ, Kofler M, Gaasch M et al. (2017) Brain temperature but not core temperature increases during spreading depolarizations in patients with spontaneous intracerebral hemorrhage. J Cereb Blood Flow Metab, 38(3): 549-558.
Schwab S, Georgiadis D, Berrouschot J et al. (2001) Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke, 32: 2033-5.
Seule MA, Muroi C, Mink S et al. (2009) Therapeutic hypothermia in patients with aneurysmal subarachnoid hemorrhage, refractory intracranial hypertension, or cerebral vasospasm. Neurosurgery, 64: 86-92.
Staykov D, Wagner I, Volbers B et al. (2013) Mild prolonged hypothermia for large intracerebral hemorrhage. Neurocrit Care, 18: 178-83.
Todd MM, Hindman BJ, Clarke WR (2005) Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med, 352: 135-45.
Van Der Worp HB, Macleod MR, Bath PM et al. (2014) EuroHYP-1: European multicenter, randomized, phase iii clinical trial of therapeutic hypothermia plus best medical treatment vs. best medical treatment alone for acute ischemic stroke. Int J Stroke, 9: 642-5.
Yao Z, You C, He M (2018) Effect and feasibility of therapeutic hypothermia in patients with hemorrhagic stroke: a systematic review and meta-analysis. World Neurosurg, 111: 404-412.e2.








