ICU Management & Practice, Volume 19 - Issue 1, 2019
The paper highlights the present clinical rationale for extravascular lung water measurement as a key to personalisation of haemodynamic therapy.
Introduction
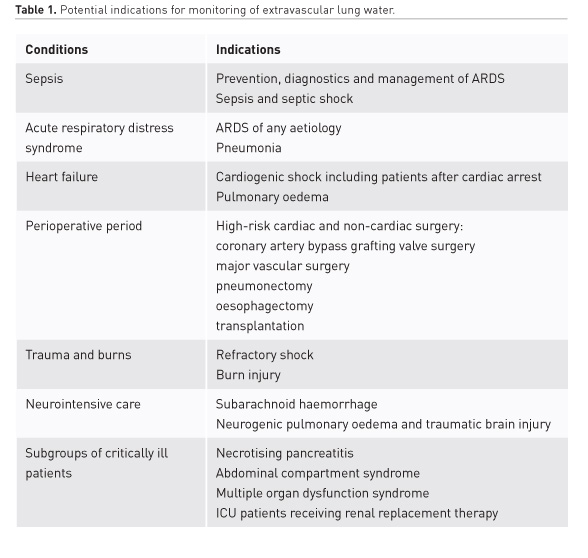
Extravascular lung water (EVLW) remains a useful guide for monitoring pulmonary oedema (PO) and vascular permeability in sepsis, acute respiratory distress syndrome (ARDS), and heart failure (Jozwiak et al. 2015; Michard 2018). Increased EVLW is associated with a high mortality and corresponds to the severity of ARDS (Sakka et al. 2002; Jozwiak et al. 2013). In addition, EVLW has a prognostic potential in shock, cardiothoracic surgery, multiple trauma, neurocritical care, and other conditions (Jozwiak et al. 2015; Tagami et al. 2018; Brown et al. 2009).
Despite a number of limitations, EVLW measured with transpulmonary thermodilution (TPTD) has documented high correlation with postmortem gravimetry and other methods in both experimental and clinical settings (Michard 2018; Tagami et al. 2010; Sakka et al. 2000; Kuzkov et al. 2007; Kirov et al. 2004). Therefore, in the twenty-first century, TPTD is still referred to as a “clinical gold standard” and a reference technique for EVLW measurement despite strong competition from non-invasive methods, including lung ultrasound, bioimpedance tomography and computed tomography (Michard 2018; Anile et al. 2017; Patroniti et al. 2005).
Importantly, EVLW can serve as a guide for personalisation of haemodynamic management. Thus, critical illness resulting in shock and tissue hypoperfusion refractory to fluid resuscitation can be considered as a target for monitoring of EVLW in combination with oxygen transport and metabolic parameters (Jozwiak et al. 2015; Monnet et al. 2018). Moreover, when integrated with treatment protocols, EVLW has a potential to improve clinical outcome (Monnet et al. 2018; Mitchell et al. 1992).
Transpulmonary thermodilution for quantification of EVLW
Methodologically, TPTD calculates cardiac output (CO) according to the Stewart–Hamilton principle, based on the analysis of thermodilution curve (Figure 1), by applying a thermal (cold saline) indicator. Primarily the TPTD monitor calculates intrathoracic thermal volume and pulmonary thermal volume by multiplying CO with the mean transit time and the down-slope time of the curve, respectively. Pulmonary thermal volume consists of pulmonary blood volume (PBV) and EVLW, representing the largest mixing volume for the indicator. The difference between intrathoracic and pulmonary thermal volumes is global end-diastolic volume (GEDV) (Sakka et al. 2000; Boussat et al. 2002). Thus, the combination of CO, EVLW, and GEDV can be a useful tool for clinical assessment of the volumetric status of the patient, especially in shock and ARDS. In addition, TPTD parameters can be used to calculate pulmonary vascular permeability index (PVPI = EVLW/PBV), in order to differentiate cardiogenic and non-cardiogenic types of PO (Jozwiak et al. 2015; Boussat et al. 2002; Kuzkov et al. 2006). The technique is suitable for application at the bedside and integrates pulse contour analysis for continuous CO monitoring and assessment of fluid responsiveness.
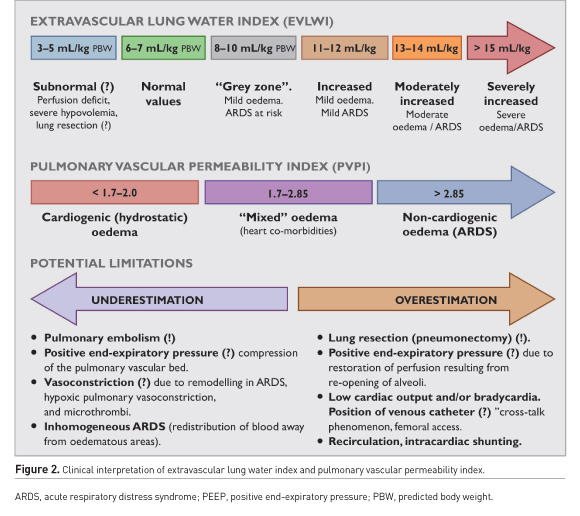
The accuracy of TPTD can be influenced by diverse changes in heat conductivity of intrathoracic structures (i.e. pleural fluids or redistribution of pulmonary blood flow), and “heat leak” of the thermal indicator (myocardium and great vessels) (Jozwiak et al. 2007; Tagami et al. 2010). Additionally, inhomogeneous PO in ARDS, recirculation of indicator due to anatomical abnormalities, and other factors might compromise the accuracy of readings (Brown et al. 2009; Kirov et al. 2004; Sakka 2013). The prognostic value of EVLW is improved by indexing to predicted body weight (EVLWPBW) (Phillips et al. 2008). Although “normal” EVLW previously was referred to as 3–7 mL/kg (Brown et al. 2009; Sakka 2013), Tagami et al. (2010) by means of postmortem gravimetry reported that normal EVLWPBW was 7.4±3.3 mL/kg (Tagami et al. 2010). Today, the best EVLW cut-off value for discriminating diffuse alveolar damage is 10 mL/kg (Tagami et al. 2018, Tagami et al. 2013), and values exceeding 15 mL/kg correspond to severe ARDS with increased mortality (Jozwiak et al. 2015, Michard 2018, Tagami et al. 2010). The values between 8 and 10 mL/kg can be considered as belonging to “a grey zone” (risk of ARDS) (Figure 2).
ARDS and hydrostatic oedema
Since PO is a hallmark of ARDS, bedside assessment of EVLW has a great potential to optimise fluid therapy and respiratory support (Jozwiak et al. 2015; Michard 2018; Sakka et al. 2002; Jozwiak et al. 2013; Tagami et al. 2018). Both EVLW and PVPI increase in non-survivors of ARDS, peaking between days 2 and 4 of the lung injury (Sakka et al. 2002; Kuzkov et al. 2006; Sakka 2013; Martin et al. 2005). In ARDS, EVLW is increased in the overwhelming majority of patients (Kuzkov et al. 2006; Le Tourneau et al. 2012). Moreover, ARDS patients with a maximum EVLW > 21 mL/kg and PVPI >3.8 have a mortality rate of approximately 70% (Jozwiak et al. 2013). In contrast, diffuse alveolar damage, which is the ultimate pathologic pattern of ARDS, was confirmed in only 45% of the patients meeting the criteria of the Berlin definition (Thille et al. 2013). Thus, EVLWPBW > 10 mL/kg is an important threshold of PO and remains an important candidate to be integrated into the current definition of ARDS (Jozwiak et al. 2015; Michard et al. 2012). This is also consistent with Kushimoto et al. (2013) demonstrating that ARDS severity by the Berlin definition was associated with EVLW of 14.7, 16.2, and 20.0 mL/kg in mild, moderate, and severe forms, respectively, while PVPI followed the same pattern with values of 2.6, 2.7 and 3.5.
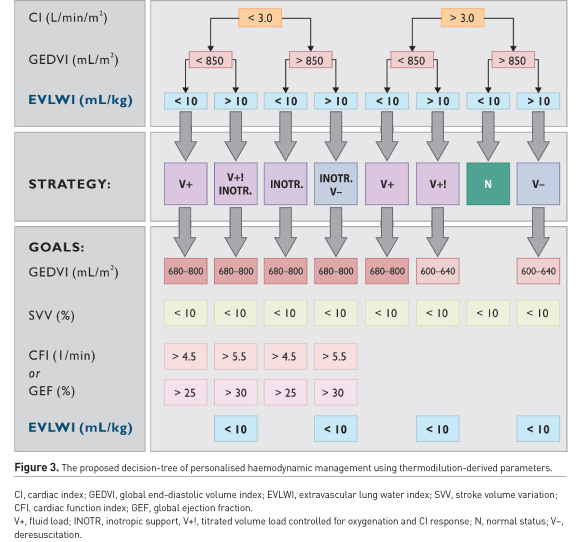
In ARDS with cardiac comorbidities, PVPI and GEDV may help to distinguish between non-cardiogenic, mixed and hydrostatic PO (Sakka et al. 2002; Tagami et al. 2018; Kushimoto et al. 2012). Recently, it has been shown that EVLW was higher in ARDS patients than in those with atelectasis or pleural effusion (Kushimoto et al. 2012). Combined with other cardiopulmonary parameters, EVLW might provide guidance for therapeutic interventions (Figure 3). In ARDS, these interventions can include the administration of albumin and furosemide, changes in PEEP, recruitment manoeuvres, prone positioning or discontinuation of respiratory support (Yagi et al. 2011; Cordemans et al. 2012; Toth et al. 2007; Smetkin et al. 2012; Chung et al. 2017). Thus, in patients with EVLW > 10 mL/kg, recruitment is less effective (Smetkin et al. 2012), although it may result in attenuation of PO (Chung et al. 2017), and EVLW > 11 mL/kg can serve as a predictor of unsuccessful weaning (Dres et al. 2014; Zeravik et al. 1990). Therefore, information about EVLW and other volumetric variables might support decisions associated with decreasing duration of respiratory support and shortening ICU and hospital stays (Brown et al. 2009; Mitchell et al. 1992: Dres et al. 2014; Zeravik et al. 1990). Moreover, a personalised management based on EVLW can reduce mortality in critically ill patients with increased EVLW, as compared to treatment guided by Swan-Ganz catheter (Eisenberg et al. 1987).
Monitoring of EVLW should also be used in patients with cardiogenic PO and circulatory shock (Tagami et al. 2012; Adler et al. 2013). The values of EVLW > 10 mL/kg associated with PVPI below 2.0 may indicate on prevalent hydrostatic mechanism of PO (Tagami et al. 2018; Kushimoto et al. 2012). In mild therapeutic hypothermia after cardiac arrest, a personalised approach to haemodynamic support aiming at EVLW ≤ 10 mL/kg, GEDV within 700–800 mL/m2, stroke volume variations (SVV) < 10%, and pulse pressure variations < 10% increases fluid load and reduces the incidence of acute kidney injury (Adler et al. 2013).
Septic shock
Haemodynamic management during septic shock and ARDS also requires a personalised approach (Figure 3). Surviving Sepsis Campaign 2016 (Rhodes et al. 2017) recommends invasive cardiovascular monitoring, and TPTD may increase the safety of fluid therapy, inotrope/vasopressor support, and mechanical ventilation in sepsis (Jozwiak et al. 2015; Tagami et al. 2018; Wang et al. 2016). It is well documented that in septic shock and ARDS, the combination of adequate fluid load during the first 6 hours and restrictive fluid strategy with cumulative zero net balance during at least 48–96 hrs, is associated with reduced mortality (Murphy et al. 2009; Sirvent et al. 2015). In septic shock, an increase in EVLW by more than 10% from baseline (exceeding 10 mL/kg), may be an incentive to limit fluid resuscitation (Cordemans et al. 2012; Aman et al. 2012) and implement protocols aimed at decreasing EVLW, shortening durations of mechanical ventilation and ICU stay and improving clinical outcome (Mitchell et al. 1992; Aman et al. 2012).
The accumulation of EVLW occurs before changes in blood gases and chest radiogram (Boussat et al. 2002; Kuzkov et al. 2006; Martin et al. 2005). In patients with sepsis, EVLW predicts progression to ARDS 2.6±0.3 days before they meet standard clinical criteria (Le Tourneau et al. 2012). Notably, Martin et al. (2005) showed that more than 50% of patients with sepsis without ARDS have increased EVLW, possibly representing subclinical lung injury.The increased EVLW in sepsis may be considered as an alarm signal for avoiding unnecessary and dangerous fluid load (Monnet et al. 2018; Rhodes et al. 2017). Moreover, in septic shock, the increase in EVLW, but not in central venous pressure, from day 1 to 3 is strongly associated with mortality and shows moderate correlation with lung injury score and gas exchange (Kuzkov et al. 2006).
What is beyond ARDS and sepsis?
Cardiac surgery
In uncomplicated off-pump coronary artery bypass grafting (CABG), perioperative haemodynamic management is accompanied by a decrease in EVLW after revascularisation (Smetkin et al. 2009). In on-pump CABG, patients with EVLW >12 mL/kg may require diuretics to attenuate PO, resulting in reduced need for vasopressors and inotropes and shortened respiratory support and ICU stay (Goepfert et al. 2007). In a controlled trial of combined CABG and aortic valve repair, TPTD-based goal-directed therapy (fluid load if SVV > 10% controlled with CO and EVLW and discontinuation of fluids when CO decreased or EVLW exceeded 12 mL/kg), reduced postoperative complications and length of ICU stay compared with the conventional approach (fluid therapy based on mean arterial and central venous pressures) (Goepfert et al. 2013).
Personalised therapy based on parameters of TPTD and oxygen transport can also be beneficial in high-risk patients after complex valve surgery. Compared with pulmonary arterial catheter, haemodynamic optimisation using GEDV, EVLW and oxygen delivery, improved haemodynamics and oxygen transport and reduced duration of postoperative respiratory support (Lenkin et al. 2012).
Non-cardiac surgery
Pulmonary oedema with EVLW >7 mL/kg is not an uncommon finding after major vascular surgery and can be caused by increased permeability in the absence of overt heart failure. Thus, EVLW might help to distinguish between ischaemia-reperfusion lung injury, atelectasis and cardiogenic PO (Groeneveld et al. 2006).
Monitoring of EVLW also might be useful in thoracic surgery including pulmonary resections, endarterectomy, and transplantation (Chau et al. 2014; Tran-Dinh et al. 2018; Naidu et al. 2009; Stéphan et al. 2017). Postpneumonectomy PO, most probably arising from excessive ventilation of the remaining lung, is a life-threatening complication (Kuzkov et al. 2007; Chau et al. 2014; Naidu et al. 2009). In both experimental and clinical settings, EVLW decreased immediately by 30% after pneumonectomy (Naidu et al. 2009), but increased significantly by 27% postoperatively (Kuzkov et al. 2007). After oesophageal resection, the increase in EVLW at 12 hours correlates with decreased oxygenation and lung compliance; therefore changes in EVLW represent a useful parameter for evaluation of respiratory status and prediction of pulmonary complications (Chau et al. 2014; Sato et al. 2007).
Trauma
In severe combined trauma with hypotension and hypoxemia, quantification of EVLW led to modifications of fluid and vasopressor support, resulting in lower fluid load and improved outcome (Pino–Sanchez et al. 2009). Lung water and other volumetric variables also provide guidance of fluid therapy in adults and children with severe burns involving more than 25–30% of body surface area (Wang et al. 2018,Kraft et al. 2013). Paediatric patients subjected to fluid resuscitation guided by CO, GEDV, and EVLW, had significantly lower fluid balance, better haemodynamic stability and decreased incidence of cardiac dysfunction and kidney injury compared with conventional monitoring (Kraft et al. 2013). To avoid tissue oedema in burns, the target values of GEDV and EVLW for fluid resuscitation in this category of patients probably should be adjusted to below-normal range (Wang et al. 2018; Aboelatta et al. 2013).
Neurocritical care
In neurocritical care, EVLW assessment might be useful to avoid neurogenic PO (Brown et al. 2009). The evaluation of EVLW together with CO and volumetric parameters has been validated as an important tool in patients with subarachnoid haemorrhage (SAH) to prevent pulmonary complications and manage life-threatening cerebral vasospasm (Mutoh et al. 2007) Following SAH, EVLW might demonstrate a biphasic increase: a cardiogenic PO due to low cardiac contractility immediately after SAH, and most likely due to hypervolemia and systemic inflammatory response from day 7 of SAH (Obata et al. 2016). In traumatic brain injury, increased EVLW might be associated with trauma severity and increased intracranial pressure and warrants further investigations (Lubrano et al. 2015; Chaari et al. 2015).
Other settings
Assessment of EVLW also can be proposed for personalisation of haemodynamic support in several other categories of patients, including necrotising pancreatitis (Huber et al. 2008), transplantation (Venkateswaran et al. 2013), multiple organ failure, and renal replacement therapy (Compton et al. 2007; Schmidt et al. 2016). It is important to bear in mind that the results of studies focusing on EVLW are strongly dependent on protocol and individualisation of target values (Trof et al. 2012).
Conclusions
Bedside assessment of EVLW has the potential to provide additional information regarding fluid status and to personalise therapy in a wide spectrum of ICU patients. Thus, EVLW plays an important diagnostic and prognostic role in sepsis, ARDS, circulatory shock, complicated perioperative period, and other high-risk patients, and was included into the current standards of their management (Cecconi et al. 2014). Assessment of EVLW should be an integral part of personalised resuscitation to improve outcome in patients at risk of PO with fluid restriction when EVLW exceeds 10 mL/kg. While TPTD remains a bedside “gold standard” in critically ill patients, evaluation of EVLW by using ultrasound has a great potential for further progress in other clinical scenarios.
Acknowledgements
Mikhail Kirov is a member of the MAB of Pulsion/Getinge and Philips. Vsevolod Kuzkov and Lars Bjertnaes declare no conflicts of interest.
Abbreviations
ARDS acute respiratory distress syndrome
CO cardiac output
CABG coronary artery bypass grafting
EVLW extravascular lung water
GEDV global end-diastolic volume
PBW predicted body weight
PBV pulmonary blood volume
PO pulmonary oedema
PVPI pulmonary vascular permeability index
SVV stroke volume variations
SAH subarachnoid haemorrhage
TPTD transpulmonary thermodilution
References:
Aboelatta Y, Abdelsalam A (2013) Volume overload of fluid resuscitation in acutely burned patients using transpulmonary thermodilution technique, J Burn Care Res, 34 (3): 349–354.
Adler C, Reuter H, Seck C, et al. (2013) Fluid therapy and acute kidney injury in cardiogenic shock after cardiac arrest, Resuscitation, 84 (2): 194–199.
Aman J, Groeneveld AB, van Nieuw Amerongen GP (2012) Predictors of pulmonary edema formation during fluid loading in the critically ill with presumed hypovolemia, Crit Care Med, 40 (3): 793–799.
Anile A, Russo J, Castiglione G, Volpicelli G (2017) A simplified lung ultrasound approach to detect increased extravascular lung water in critically ill patients. Crit Ultrasound J, 9 (1): 13.
Boussat S, Jacques T, Levy B, et al. (2002) Intravascular volume monitoring and extravascular lung water in septic patients with pulmonary edema, Intensive Care Med, 28 (6): 712–718.
Brown LM, Liu KD, Matthay MA (2009) Measurement of extravascular lung water using the single indicator method in patients: research and potential clinical value, Am J Physiol Lung Cell Mol Physiol, 297 (4): L547–L558.
Cecconi M, De Backer D, Antonelli M, et al. (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine, Intensive Care Med, 40 (12):1795-1815.
Chaari A, Chtara K, Toumi N, et al. (2015) Neurogenic pulmonary edema after severe head injury: a transpulmonary thermodilution study, Am J Emerg Med, 33 (6): 858.e1–3.
Chau EH, Slinger P (2014) Perioperative fluid management for pulmonary resection surgery and esophagectomy, Semin Cardiothorac Vasc Anesth, 18 (1): 36–44.
Chung FT, Lee CS, Lin SM, et al. (2017) Alveolar recruitment maneuver attenuates extravascular lung water in acute respiratory distress syndrome, Medicine (Baltimore), 96 (30): e7627.
Compton F, Hoffmann C, Zidek W et al. (2007) Volumetric hemodynamic parameters to guide fluid removal on hemodialysis in the intensive care unit, Hemodialysis International, 11 (2): 231–237.
Cordemans C, De Laet I, Van Regenmortel N, et al. (2012) Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: a pilot study looking at the effects of PAL-treatment, Ann Intensive Care, 5 (2) Suppl 1: S15.
Dres M, Teboul JL, Anguel N et al. (2014) Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema, Crit Care Med, 42 (8): 1882–1889.
Eisenberg PR, Hansbrough JR, Anderson D, Schuster DP (1987) A prospective study of lung water measurements during patient management in an intensive care unit, Am Rev Respir Dis, 136 (3): 662–668.
Goepfert MS, Reuter DA, Akyol D et al. (2007) Goal-directed fluid management reduces vasopressor and catecholamine use in cardiac surgery patients, Intensive Care Med, 33 (1): 96–103.
Goepfert MS, Richter HP, Zu Eulenburg C, et al. (2013) Individually optimized hemodynamic therapy reduces complications and length of stay in the intensive care unit: a prospective, randomized controlled trial, Anesthesiology, 119 (4): 824–836.
Groeneveld AB, Verheij J, van den Berg FG, et al. (2006) Increased pulmonary capillary permeability and extravascular lung water after major vascular surgery: effect on radiography and ventilatory variables, Eur J Anaesthesiol, 23 (1): 36–41.
Huber W, Umgelter A, Reindl W, et al. (2008) Volume assessment in patients with necrotizing pancreatitis: a comparison of intrathoracic blood volume index, central venous pressure, and hematocrit, and their correlation to cardiac index and extravascular lung water index, Crit Care Med, 36 (8): 2348–2354.
Jozwiak M, Teboul J–L, Monnet X (2015) Extravascular lung water in critical care: recent advances and clinical applications, Ann Intensive Care, 5 (1): 38.
Jozwiak M, Silva S, Persichini R, et al. (2013) Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome, Crit Care Med, 41 (2): 472–480.
Kirov MY, Kuzkov VV, Kuklin VN, et al. (2004) Extravascular lung water assessed by transpulmonary single thermodilution and post mortem gravimetry in sheep, Crit Care, 8: R451–R458.
Kraft R, Herndon DN, Branski LK, et al. (2013) Optimized fluid management improves outcomes of pediatric burn patients, J Surg Res, 181 (1): 121–128.
Kushimoto S, Endo T, Yamanouchi S, et al. (2013) Relationship between extravascular lung water and severity categories of acute respiratory distress syndrome by the Berlin definition, Crit Care, 17 (4): R132.
Kushimoto S, Tairo Y, Kitazawa Y, et al. (2012) The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: a prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome, Crit Care, 16 (6): R232
Kushimoto S, Taira Y, Kitazawa Y, et al.; PiCCO Pulmonary Edema Study Group. (2012). The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: a prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome, Crit Care,16 (6): R232.
Kuzkov VV, Suborov EV, Kirov MY, et al. (2007) Extravascular lung water after pneumonectomy and one-lung ventilation in sheep, Crit Care Med, 35 (6): 1550–1559.
Kuzkov VV, Kirov MY, Sovershaev MA et al. (2006) Extravascular lung water determined with single transpulmonary thermodilution correlates with the severity of sepsis-induced acute lung injury, Crit Care Med, 34 (6): 1647–1653.
Kuzkov V, Uvarov D, Kruchkov D, et al. (2007) Determination of extravascular lung water after pneumonectomy and lung resection, Acta Anaesth Scand, 51 (Suppl. 118):10 (abstract).
Lenkin AI, Kirov MY, Kuzkov VV, et al. (2012) Comparison of goal-directed hemodynamic optimization using pulmonary artery catheter and transpulmonary thermodilution in combined valve repair: a randomized clinical trial, Crit Care Res Pract: article ID 821218.
Le Tourneau JL, Pinney J, Phillips CR (2012) Extravascular lung water predicts progression to acute lung injury in patients with increased risk, Crit Care Med, 40 (3): 847–854.
Lubrano R, Elli M, Stoppa F, Di Traglia M, et al. (2015). Variations of the blood gas levels and thermodilutional parameters during ICP monitoring after severe head trauma in children, Childs Nerv Syst, 31 (8): 1273–1281.
Martin GS, Eaton S, Mealer M, Moss M (2005) Extravascular lung water in patients with severe sepsis: a prospective cohort study, Crit Care, 9: R74–R82.
Michard F (2018) Lung water assessment: from gravimetry to wearables, J Clin Monit Comput, Epub ahead of print.
Michard F, Fernandez–Mondejar E, Kirov M, et al. (2012) A new and simple definition for acute lung injury, Crit Care Med, 40 (3): 1004–1007.
Mitchell JP, Schuller D, Calandrino FS, Schuster DP (1992) Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization, Am Rev Respir Dis, 145 (5): 990–998.