Accelerated Partial Breast Irradiation (APBI) with Balloon Brachytherapy
Hologic’s targeted radiation therapy is a breakthrough early-stage breast cancer treatment that can offer an alternative to whole breast radiation or a mastectomy.
Balloon brachytherapy solutions directly target the lumpectomy cavity, where the cancer is most likely to recur, sparing healthy tissues and organs from the damaging effects of radiation.1-2
Hologic offers a choice between two systems: MammoSite® and Contura® systems.
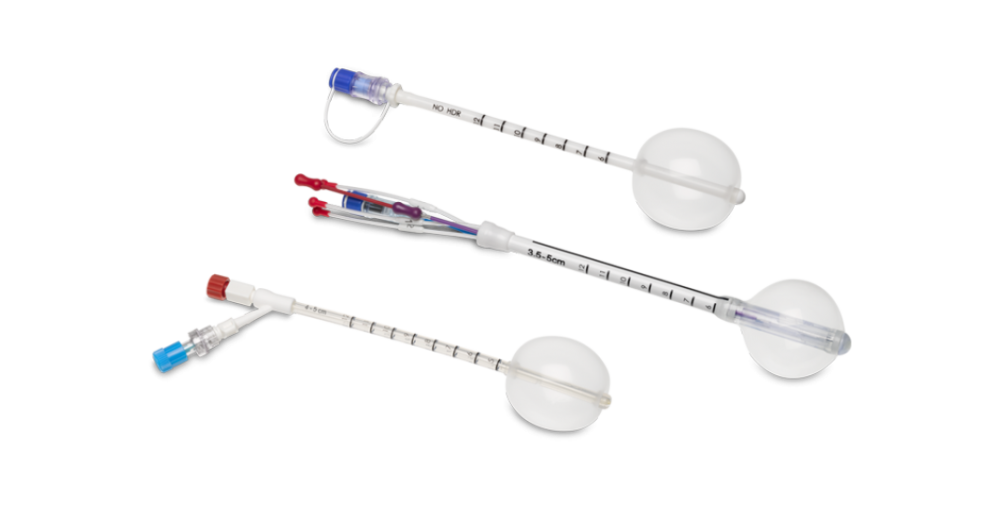
MammoSite Targeted Radiation Therapy Solutions
The MammoSite system is Hologic’s legacy balloon brachytherapy system. It’s available in two configurations: single-lumen and multi-lumen catheters. The multi-lumen catheter has four offset lumens to allow for dosimetric optimization to minimize skin dose. This balloon also has a purple keyed stylet that helps you position the balloon after implantation.
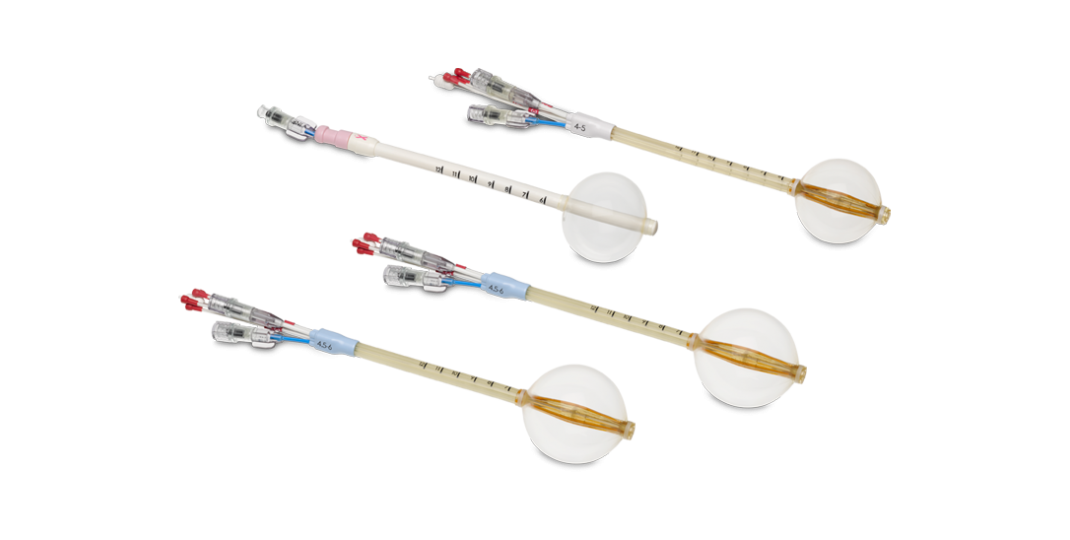
Contura Multi-lumen Balloon Solutions
Contura multi-lumen balloon is a single insertion balloon brachytherapy applicator available in two sizes and two shaft configurations (standard and FLEX). With five treatment lumens, you can shift the isodose curve from the chest wall. There are two vacuum ports on the proximal and distal ends of the balloon that allow you to remove fluid or air to facilitate tissue conformance to the balloon.
Both MammoSite and Contura multi-lumen balloon brachytherapy applicators are the only single-insertion APBI technologies that meet or exceed all B-39 dosimetric guidelines.
As such, they allow you to easily satisfy the current clinical recommendation that the dose to the skin be maintained to less than 125% of the prescription dose.3-5
Clinical Data
With seven years of positive clinical evidence, and over 90,000 women successfully treated, you can feel confident recommending this solution to your patients.2,6,8
The final analysis of the American Society of Breast Surgeons (ASBS) MammoSite Breast Brachytherapy Registry Trial (N=1440) confirmed the excellent results of treatment efficacy, cosmesis and toxicity. The study also concluded that these results are similar and compared favorably with other forms of APBI and whole breast irradiation with similar follow-up period.2
- Excellent/good cosmesis was observed in:
91.3% of patients at 5 years.
90.5% of patients at 6 years.
90.6% of patients at 7 years.
- Overall rate of fat necrosis was 2.5% with an infection rate of 9.6% and few late toxicity events beyond 2 years.
- Overall symptomatic seroma rate was 13.4% and 0.6% beyond 2 years.
- Ipsilateral breast tumor recurrence (IBTR) was developed in 41 cases (2.8%) for a 5-year actuarial rate of 3.8 % (3.7% for IBC and 4.1% for DCIS).
The Five-year Results of the Initial Clinical Trial of MammoSite Balloon Brachytherapy for Partial Breast Irradiation in Early-Stage Breast Cancer involving 43 patients7 shows:
- No local recurrences.
- 83.3% of patients had good/excellent cosmetic results.
- 100% of patients would recommend MammoSite targeted radiation therapy to a friend or family member.
- 100% of patients would use MammoSite targeted radiation therapy if they had to do it over.
The safety and effectiveness of the MammoSite® radiation therapy system (RTS), MammmoSite® ML radiation therapy system and the Contura® applicator as a replacement for whole breast irradiation in the treatment of breast cancer has not been established.
More information can be found here
Loading...