Oncologic imaging is pivotal in assessing how cancer patients respond to treatment. Accurate and consistent evaluation is crucial for determining prognosis, adjusting therapeutic strategies, and ultimately improving patient outcomes. Over the years, various response assessment criteria have been developed to standardise these evaluations, with RECIST 1.1 being the most widely used framework. However, the evolution of cancer therapies, particularly the advent of immunotherapy, has necessitated the development of more nuanced criteria like iRECIST, mRECIST, and Choi criteria. These ESR Essentials practice recommendations explore these response assessment criteria, highlighting their application in clinical trials and routine practice.
RECIST 1.1 Criteria: The Gold Standard
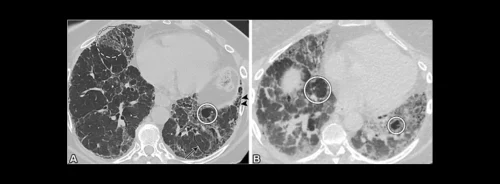
RECIST (Response Evaluation Criteria in Solid Tumours) 1.1, established in 2009, remains the cornerstone for evaluating treatment response in patients with solid tumours. This framework, which builds upon the original RECIST 1.0, primarily relies on unidimensional measurements of tumour lesions. The criteria define specific thresholds for categorising patient responses into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), based on changes in the sum of the longest diameters (SLD) of target lesions.
In clinical trials, RECIST 1.1 ensures consistency and reliability in reporting outcomes, thus allowing for meaningful comparisons across studies. Despite its robustness, RECIST 1.1 has limitations, particularly in scenarios where tumour size alone does not accurately reflect treatment efficacy. For example, some therapies might cause tumours to change density or vascularisation without significant size reduction. As a result, supplementary criteria are sometimes necessary to capture the full spectrum of tumour response.
iRECIST: Adapting to Immunotherapy
The rise of immunotherapy, which harnesses the body’s immune system to combat cancer, has revolutionised oncology but also introduced new challenges for response assessment. Traditional criteria like RECIST 1.1 may misclassify immune-related phenomena such as pseudoprogression—where tumours initially appear to grow before shrinking—as disease progression. To address this, iRECIST was developed as a modified version of RECIST 1.1, tailored specifically for immune-based therapies.
iRECIST introduces the concept of unconfirmed progressive disease (iUPD), which requires further verification before classifying a patient's response as progressive disease (iCPD). This approach allows for observing immune-related responses over time, acknowledging that initial tumour growth may not always indicate treatment failure. By distinguishing between actual progression and pseudoprogression, iRECIST provides a more accurate framework for evaluating immunotherapy's efficacy in clinical trials, ensuring that patients are not prematurely removed from potentially beneficial treatments.
mRECIST and Choi Criteria: Tailoring to Specific Cancers
Hepatocellular carcinoma (HCC) and gastrointestinal stromal tumours (GIST) present unique challenges in response assessment, necessitating specialised criteria. The mRECIST (modified RECIST) criteria were explicitly developed for HCC, often involving local therapies and non-cytotoxic systemic treatments. Unlike traditional RECIST criteria, mRECIST focuses on measuring the viable, enhancing portions of tumours rather than overall tumour size. This approach is particularly useful for HCC, where the presence of necrosis or non-enhancing tissue within the tumour can complicate response evaluation.
Similarly, the Choi criteria were devised to assess GISTs treated with targeted therapies like imatinib. These criteria consider both the size and density of the tumour, acknowledging that effective treatment may lead to reduced tumour density without a corresponding decrease in size. By incorporating these additional parameters, the Choi criteria offer a more nuanced evaluation of treatment response, which is particularly valuable in managing GISTs.
Conclusion
The landscape of oncologic imaging and response assessment is continuously evolving to keep pace with advancements in cancer therapy. While RECIST 1.1 remains a foundational tool, the development of iRECIST, mRECIST, and Choi criteria reflects the need for more specialised approaches in evaluating treatment efficacy. These criteria not only enhance the precision of response assessments in clinical trials but also offer valuable guidance for routine clinical practice. As cancer treatment becomes increasingly personalised, the ongoing refinement of imaging criteria will ensure patients receive the most appropriate and effective care.
Source: European Radiology
Image Credit: iStock