ICU Management & Practice, Volume 23 - Issue 2, 2023
Vasopressors and inotropes are frequently used in intensive care units. With a special focus on recent studies, this article summarises the key messages in the management of patients requiring inotropes and vasopressors.
Introduction
Cardiac output (CO) is a key determinant of oxygen delivery. Low cardiac output syndrome (LCOS) causes organ dysfunction, prolonged hospital stay, and reduces survival in perioperative settings and in critical illness (Algarni et al. 2011; Maganti et al. 2010; Maganti et al. 2005; Lomivorotov et al. 2017; Zangrillo et al. 2020). Ultimately, the inability of the circulatory system to match oxygen demand is considered the main pathophysiological cause underlying the development of multi-organ failure and death (Schoemaker et al. 1988; Vincent et al. 2012). When heart function is incapable of providing enough CO to support tissues metabolic demands, inotropes can be administered with the goal of improving cardiac contractility and, therefore, restore and maintain an adequate oxygen delivery (Fellahi et al. 2013; Francis et al. 2014).
Similarly, maintenance of an adequate mean arterial pressure (MAP) is widely accepted as fundamental to ensure end-organ perfusion, and most professional guidelines recommend starting vasopressor administration when fluid resuscitation alone is unable to restore MAP (Evans et al. 2021; Van Diepen et al. 2017; Chioncel et al. 2020; Møller et al. 2018; Møller et al. 2016).
As a consequence, every clinician caring for patients with cardiovascular dysfunction is familiar with inotropes and vasopressors. Vasoactive medications are typically used in cardiogenic shock, septic shock, acute heart failure, and patients undergoing cardiac or high-risk non-cardiac surgery. In general, every critically ill patient may require some degree of haemodynamic support.
Inotropes and vasopressors have been administered for decades to patients with cardiovascular failure, and, as many other interventions (e.g. blood products transfusion, intra-aortic balloon pump), entered in routine clinical practice well before development of the evidence-based medicine concept. Accordingly, their safety and efficacy have never been formally tested. We will summarise recent evidence regarding use of inotropes and vasopressors in critically ill patients.
Haemodynamic and Side Effects of Vasoactive Agents
Every available inotropic agent increases cardiac contractility to a variable degree. Some agents such as epinephrine and dobutamine also have chronotropic effect, with the increase in heart rate further contributing to CO increase. Effect on vascular tone is variable, with some agents also having vasoconstrictor effect (inoconstrictors or inopressors) and others having a vasodilator effect (inodilators). As a result, the net effect of the different molecules on blood pressure depends on relative and absolute patient volume status and might be difficult to be predicted.
Pure vasoconstrictors (Table 1) (Francis et al. 2014; Gillies et al. 2005; Overgaard and Dzavik 2008; Bangash et al. 2012; Jentzer et al. 2015; Annane et al. 2018; Maack et al. 2019; Belletti et al. 2022) such as phenylephrine or vasopressin generally increase MAP, and often reduce CO even if their effect on CO depends on cardiac function, subsequent effects on heart rate and stressed and unstressed volume (Funk et al. 2013a; Funk et al. 2013b; Hamzaoui et al. 2018; Thiele et al. 2011a; Thiele et al. 2011b).
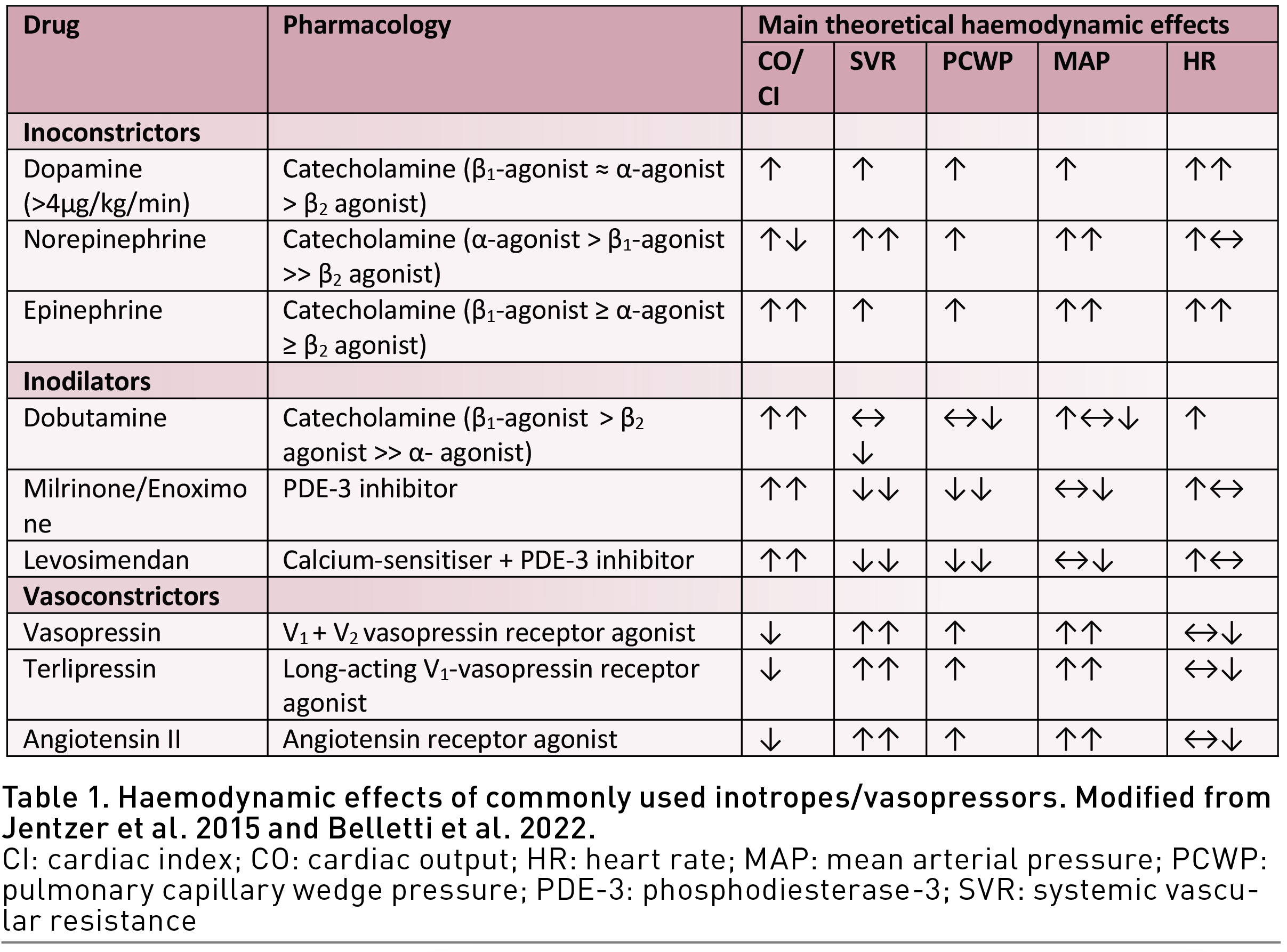
Despite the proven positive haemodynamic effects, inotropes and vasopressors are not free from side effects. The most frequently described are tachycardia, ventricular and supraventricular arrhythmias, and [with the possible exception of levosimendan (Papp et al. 2012; Nieminen et al. 2013)] increase in myocardial oxygen consumption (Fellahi et al. 2013; Arrigo and Mebazaa 2015; Schmittinger et al. 2012). In addition, inodilator agents may also cause severe hypotension (Nieminen et al. 2013; Arrigo et al. 2015), while inoconstrictors and pure vasoconstrictors may cause limb and mesenteric ischaemia (Anantasit et al. 2014).
Catecholamines, the most frequently used vasoactive agents, also have a wide range of effects on respiratory, endocrine, immunological, gastrointestinal, and coagulation system that could be detrimental when adrenergic stimulation becomes excessive (Andreis and Singer 2016; Dünser and Hasibeder 2009; Belletti et al. 2020; Freestone et al. 2012). Increase in cardiomyocytes apoptosis has been described and may be particularly important in patients with a limited cardiovascular reserve (Rona 1985; Singh et al. 2001; Felker et al. 2003). Cardiac side effects have been reported in almost half of patients receiving catecholamine therapy (Schmittinger et al. 2012).
Between the end of the 80s and the early 90s, several large RCTs demonstrated reduction in survival in patients with chronic, stable heart failure treated with daily administration of inotropes, regardless of molecule tested (Packer et al. 1991; Xamoterol in Severe Heart Failure Study Group 1990; Cohn et al. 1998). Since then, side effects of inotropes are supposed to outweigh the positive haemodynamic effect of these drugs in patients in a stable clinical condition.
More recently, several authors have raised concerns regarding safety of inotropes also in acute clinical settings. Several observational trials reported an association between inotropes administration and poor survival in acute heart failure (Abraham et al. 2005; Mebazaa et al. 2011; Mortara et al. 2014; O'Connor et al. 1999; Costanza et al. 2007; Rossinen et al. 2008; Kalogeropoulos et al. 2014), cardiac surgery (Fellahi et al. 2009; Shahin et al. 2011; Nielsen et al. 2014) and septic shock (Wilkman et al. 2013), although other observational trials did not find a similar association (Williams et al. 2011). In addition, some meta-analyses also highlighted a trend towards increased mortality when catecholamines are administered in patients with heart failure (Thackray et al. 2002; Tacon et al. 2012).
Despite evidence from observational trials, there is currently no randomised clinical trial demonstrating that inotropes administration increase mortality in settings other than chronic stable heart failure (Belletti et al. 2015). However, it should be acknowledged that there are no trials randomising haemodynamically unstable patients to inotropes/vasopressors versus no vasoactives.
Some indirect evidence may derive from trials investigating timing and intensity of vasoactive treatment, for example liberal (or higher) versus restrictive (or lower) haemodynamic targets (e.g. high vs low MAP, high vs low CO). Indeed, mRCTs comparing higher versus lower MAP targets (and hence greater versus lower exposure to exogenous vasopressors) for septic shock patients showed no difference in mortality, although trends towards lower mortality but higher rate of AKI were generally observed in the low-MAP groups (Asfar et al. 2014; Lamontagne et al. 2020). Similarly, a recent large mRCT compared restrictive (prioritising lower intravenous fluid volumes and vasopressors) versus a liberal (prioritising higher volumes of intravenous fluids before vasopressor use) fluid strategy did not show mortality or serious adverse events difference between the two groups (NHLBI Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network 2023). Few, small RCTs assessing different timing of norepinephrine administration (early versus delayed) in patients with septic shock have been performed, suggesting greater benefit with early norepinephrine administration (Permpikul et al. 2019; Elbouhy et al. 2019). Trials comparing supraphysiologic CO or oxygen delivery targets versus standard treatment in critically ill patients showed no additional benefit (Gattinoni et al. 1995), or even harm (Hayes et al. 1994) associated with higher intensity treatment.
Collectively, these studies suggested that, in critically ill patients, higher targets (and hence greater use of interventions including fluids, vasopressors, and inotropes) are generally not necessary and sometimes may be harmful (Asfar et al. 2014; Lamontagne et al. 2020; Gattinoni et al. 1995; Hayes et al. 1994; Hernández et al. 2019).
A large number of RCTs investigated the effect of perioperative goal-directed haemodynamic therapy in various types of surgery (Jessen et al. 2022; Brienza et al. 2019; Giglio et al. 2021). There is agreement that goal-directed haemodynamic therapy (a bundle of vasopressors/inotropes, fluids, and blood products, to target tissue perfusion or haemodynamic targets) in the first hours after surgical procedures reduces complications in high-risk surgery patients, while improvement in survival remains debated (Giglio et al. 2021; Hamilton et al. 2011; Cecconi et al. 2013; Pearse et al. 2014; Osawa et al. 2016). Of note, goal-directed haemodynamic therapy may also reduce cardiac complications, which, theoretically, can increase when administering catecholamines (Arulkumaran et al. 2014). Nevertheless, the question of whether inotropes in addition to fluids provide increasing benefit remains open according to some authors (Nielsen and Algotsson 2015).
Specific Molecules
In this section, we will review the latest evidence on specific inotropes/vasopressors, with a focus on most recent or largest RCTs and meta-analyses. A detailed review of pharmacology of inotropes and vasopressors is available elsewhere (Fellahi et al. 2013; Francis et al. 2014; Overgaard and Dzavik 2008; Bangash et al. 2012; Jentzer et al. 2015; Annane et al. 2018; Maack et al. 2019; Belletti et al. 2022) and summarised in Table 2.
Catecholamines
First-line vasoactive agents are usually represented by catecholamines which are infused to patients who are unstable under the haemodynamic point of view, with guidelines and experts consensus suggesting their use in different settings (Evans et al. 2021; Van Diepen et al. 2017; Chioncel et al. 2020; McDonagh et al. 2021; Mebazaa et al. 2010; Mebazaa et al. 2016; Mebazaa et al. 2018; Scheeren et al. 2021) and with epinephrine, dobutamine, dopamine, and norepinephrine being the most frequently used (Jentzer et al. 2015).
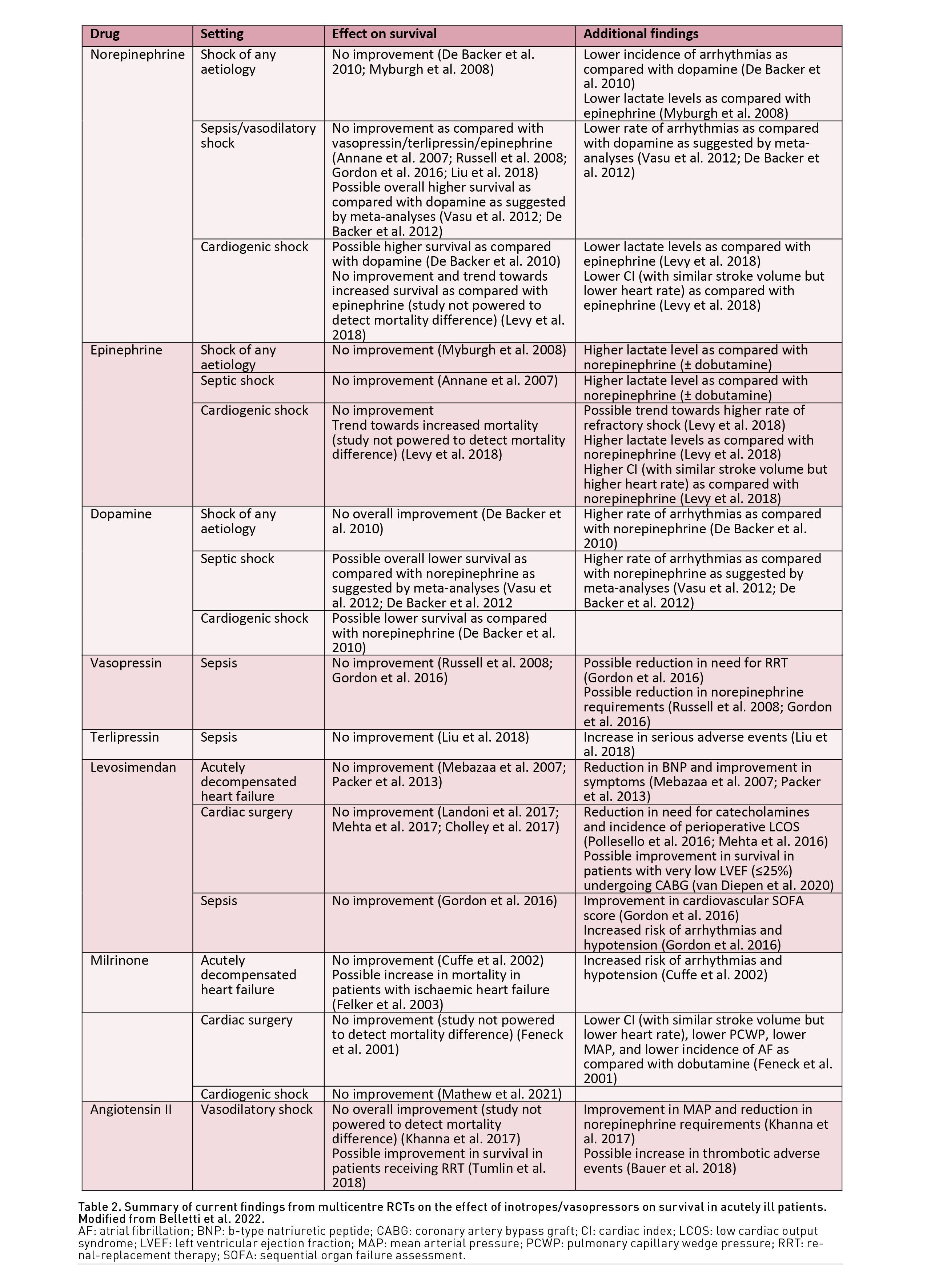
Noradrenaline is the first-line vasopressor recommended to rise MAP in all clinical contexts by most available guidelines (Evans et al. 2021; Chioncel et al. 2020; McDonagh et al. 2021). An interesting observational study performed in the United States assessed patient outcome during a period of norepinephrine shortage and documented that unavailability of noradrenaline resulted in reduced survival despite use of alternative agents such as vasopressin, dopamine and phenylephrine (Vail et al. 2017). Norepinephrine has been studied in several multicentre RCTs against dopamine, vasopressin, and epinephrine (De Backer et al. 2010; Annane et al. 2007; Myburgh et al. 2008; Levy et al. 2018; Russell et al. 2008; Gordon et al. 2016). Collectively, these studies showed no clear improvement in survival when using norepinephrine over other agents. In the Sepsis Occurrence in Acutely Ill Patients II (SOAP-II) trial, 1679 patients requiring vasopressors were randomised to receive norepinephrine or dopamine (De Backer et al. 2010). In the overall study population, there was no difference in 28-days or 1-year survival. Norepinephrine was associated with lower rate of arrhythmias in the overall population, and a higher survival rate in the subgroup of cardiogenic shock patients. Mortality reduction associated with norepinephrine use as compared with dopamine has been confirmed in meta-analyses of RCTs mostly including septic shock trials (Vasu et al. 2012; De Backer et al. 2012).
Of note, there is little awareness overall that norepinephrine is marketed under different salt preparations (e.g. tartrate, hydrochloride) with different equivalent potency to the referral product (norepinephrine base) (Leone et al. 2022; Mongardon et al. 2023; Bitton et al. 2022), while the referral product is not marketed at all. Clinical scientists and experts should be aware of this and overtly state whether they refer to norepinephrine base or other formulations when presenting trial results or recommendations.
Epinephrine is commonly used in critically ill patients as second-line agent or alternative vasopressor, especially in low-resource settings (Evans et al. 2021). In clinical practice, epinephrine is generally considered more an inotrope than a vasoconstrictor, while the opposite is true for norepinephrine. Accordingly, several clinicians prefer to use epinephrine in patients with myocardial dysfunction and are scared of noradrenaline which might increase afterload and decrease CO. However, recent observational studies noted that epinephrine is used in cardiogenic shock patients with high mortality (Léopold et al. 2018; Tarvasmäki et al. 2016). On the contrary, when pooling RCTs only no evidence of increased mortality was noted in patients randomised to receive epinephrine (Belletti et al. 2020). The study, however, also underlined the very limited number of RCTs performed in the setting of cardiogenic shock, and the overall limited numbers of RCTs investigating epinephrine as vasopressor outside the context of cardiopulmonary resuscitation (Belletti et al. 2020; Belletti et al. 2018).
In a recent RCT by Levy et al. (2018), epinephrine was compared against nor-epinephrine in patients (n=57) with cardiogenic shock due to acute myocardial infarction. The trial was interrupted early for safety issues due to a higher rate of refractory shock and a trend towards increased mortality in the epinephrine group. Haemodynamic data collected in the trial showed that epinephrine increased CO more than norepinephrine. However, this was driven by an increase in heart rate, while measured stroke volume remained similar between the two groups. This might be relevant in the context of myocardial ischaemia, as heart rate is a major determinant of myocardial oxygen consumption. It should be noted that very high dose of catecholamines (0.6-0.7 μg/kg/min) were used in this trial. Subtle haemodynamic effects may become more relevant at lower doses (e.g. 0.1-0.2 μg/kg/min). The trial has some limitations, such as higher baseline lactate levels in the epinephrine group and including lactate as a component of a safety outcome of refractory shock (despite the well-known effect of epinephrine on lactate). Nevertheless, these results challenge the notion that norepinephrine is detrimental in patients with myocardial dysfunction and provide a background for its use and further studies in this clinical setting (van Diepen 2018).
Vasopressin and terlipressin
Vasopressin is a pure vasoconstrictor and has been increasingly used in recent years as an alternative or an adjunct to norepinephrine.
The Vasopressin and Septic Shock Trial (VASST) trial, published in 2008, was the first, large RCT comparing vasopressin versus norepinephrine in septic shock (Russell et al. 2008). In this study, 778 patients with septic shock requiring 5 μg/min of norepinephrine were randomised to receive vasopressin or norepinephrine on top of open-label vasopressors.
The study showed that vasopressin improves MAP and reduces requirements of concomitant vasopressors but does not improve survival. However, subgroup and post-hoc analyses suggested that vasopressin, especially in combination with steroids, may reduce mortality and rate of acute kidney injury in patients with less severe shock (Gordon et al. 2010; Russell et al. 2009). This hypothesis was subsequently tested in a 2×2 factorial trial investigating the effect of vasopressin and hydrocortisone in early septic shock (Vasopressin vs Norepinephrine as Initial Therapy in Septic Shock [VANISH]) (Gordon et al. 2014).
This RCT, enrolling 409 patients with early septic shock (Gordon et al. 2016), showed no difference in survival, a lower rate of renal-replacement therapy (RRT) in the vasopressin group (although driven by reduction in RRT only in non-survivors), and a higher rate of digital and myocardial ischaemia in the vasopressin group. Taken together, these data suggest that vasopressin effectively reduces norepinephrine requirements and increases MAP, but with no significant effects on major outcomes. The only potential benefit may be on renal outcomes, as also suggested by a recent single-centre RCT performed in the setting of post-cardiotomy vasoplegic shock (Hajjar et al. 2017). This study (Vasopressin versus Norepinephrine in Patients with Vasoplegic Shock after Cardiac Surgery [VANCS]) showed a lower rate of AKI and atrial fibrillation in the vasopressin group, with no difference in survival or rate of adverse events.
Similarly, terlipressin (a long-acting analogue of vasopressin), despite some promising early results (Belletti et al. 2015; Serpa Neto et al. 2012; Avni et al. 2015; Kochkin et al. 2021), failed to show improvement in outcomes in a recent mRCT of 617 patients (Liu et al. 2018). On the contrary, terlipressin use increased rate of serious adverse events, and in particular rate of digital ischaemia.
Phosphodiesterase 3-inhibitors
Phosphodiesterase-3 inhibitors are inodilators frequently used as inotropic agents in patients with LCOS, especially in acute heart failure, of cardiac surgery, and in patients receiving chronic beta-blocker therapy (McDonagh et al. 2021; Bignami et al. 2016; Kastrup et al. 2007; Lowes et al. 2001; Metra et al. 2002). They are generally considered as an alternative to catecholamines, or as a synergic agent in patients requiring high-dose inotropic support.
In the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study, patients with acutely decompensated heart failure but without shock were randomised to receive milrinone or placebo (Cuffe et al. 2002; Cuffe et al. 2000). Patients randomised to milrinone had a higher rate of hypotension and arrhythmias, while rate of mortality and other major outcomes remained comparable. In addition, an interesting post-hoc analysis suggested that milrinone may be beneficial in patients with non-ischaemic heart failure, while it may worsen outcome in patients with ischaemic heart failure (Felker et al. 2003).
Another multicentre RCT performed in the setting of cardiac surgery compared milrinone versus dobutamine in patients with perioperative LCOS (Feneck et al. 2001). The study focused on haemodynamic parameters and was not powered to assess clinical endpoints. It showed that dobutamine administration was associated with higher cardiac index (driven by a greater increase in heart rate), higher MAP, and higher incidence of atrial fibrillation, while milrinone was associated with greater decrease in pulmonary capillary wedge pressure (PCWP).
A single-centre study published in 2021 randomised 192 patients with cardiogenic shock {Society of Cardiovascular Angiography and Interventions [SCAI]-stage B or higher (Baran et al. 2019)} to receive dobutamine or milrinone as primary inotropic agent (Dobutamine Compared to Milrinone [DOREMI] study) (Mathew et al. 2021). The authors found no difference in terms of mortality, adverse events, haemodynamic parameters or need for vasopressors. Overall, these studies confirm the haemodynamic efficacy of milrinone in terms of CO increase and vasodilation, but also demonstrate neutral effects on major clinical outcomes, as compared with catecholamines.
Interestingly, an experimental study assessing haemodynamic effect of milrinone and catecholamines in conditions independent from pre- and afterload, showed that milrinone may have no direct inotropic effect contrary to dobutamine. Accordingly, the authors hypothesised that the increase in cardiac output observed with PDE-3 inhibitors may be related to their pre- and afterload modulation properties, rather than a direct increase in cardiac contractility (DeWitt et al. 2016). This might also explain the greater effect on PCWP observed as compared with dobutamine.
Levosimendan
Levosimendan is a relatively new inodilator agent acting as a calcium-sensitiser and PDE-3 inhibitor. It has been extensively studied and indeed is the most frequently investigated inotropic agent ever, with more than 100 RCTs including almost 10000 patients (Belletti et al. 2015). Several early RCTs and meta-analyses of RCTs suggested that levosimendan administration could improve survival in a wide variety of clinical settings (Pollesello et al. 2016).
From mid 2000s, several high-quality, large mRCTs investigated the effect of levosimendan on major outcomes in the settings of acute heart failure, cardiac surgery and sepsis (Landoni et al. 2017; Zangrillo et al. 2016; Mehta et al. 2017; Mehta et al. 2016; Orme et al. 2014; Gordon et al. 2016; Cholley et al. 2017; Caruba et al. 2016; Mebazaa et al. 2007; Packer et al. 2013). Contrary to meta-analyses and early results, all these studies failed to show a convincing beneficial effect of levosimendan on mortality or other major clinical outcomes. These studies confirmed that levosimendan administration leads to reduction in need for other concomitant inotropes and higher rate of hypotension (results that are consistent with its inodilator effect) and arrhythmias. One post-hoc analysis of a cardiac surgery RCT suggested a potential beneficial effect for the limited group of patients with very low left ventricular ejection fraction undergoing coronary artery bypass graft surgery, when levosimendan is administered prophylactically (van Diepen et al. 2020). Another post-hoc analysis in the setting of acute heart failure suggested greater benefit for patients on chronic beta-blocker therapy, as compared with dobutamine (Mebazaa et al. 2009). These findings should be confirmed in adequately powered trials.
Interestingly, while traditionally considered a calcium-sensitiser, some experimental studies challenged this view and suggested that the haemodynamic effects of levosimendan are almost exclusively related to its effect as inhibitor of the PDE-3 (Ørstavik et al. 2014), and potentially to its effect on vascular K+-ATP channels (Maack et al. 2019), while the calcium-sensitising properties exert a very limited effect (Ørstavik et al. 2014).
Angiotensin II
Angiotensin II is a vasopressor that has been suggested as a catecholamine-sparing agent for patients with vasodilatory shock and increasingly studied in recent years .
In the largest and most recent mRCT performed, 344 patients with vasodilatory shock requiring > 0.2 µg/kg/min of norepinephrine and with a normal cardiac index were randomised to receive angiotensin II or placebo on top of open-label norepinephrine (Khanna et al. 2017). The study showed that angiotensin II does increase MAP and reduces need for concomitant norepinephrine. The study was underpowered to detect major outcome differences. However, no hints for benefit or harms were reported. A post-hoc analysis investigating patients receiving RRT at randomisation suggested that angiotensin II may improve survival and renal recovery in this subgroup of patients (Tumlin et al. 2018). However, these findings require further confirmation in adequately powered studies. Of note, some authors suggested that angiotensin II use may be associated with an increased rate of delirium, LCOS, thrombotic events, and fungal infections (Thiele et al. 2011a; Thiele et al. 2011b; Bauer et al. 2018).
Future Directions
Mechanical circulatory support (MCS) is increasingly used in recent years, in particular in the setting of acute heart failure/cardiogenic shock (Combes et al. 2020; Rihal et al. 2015; Atkinson et al. 2016). Interestingly, MCS is increasingly used also in unconventional settings including sepsis (Bréchot et al. 2020) and high-risk surgical/interventional procedures (Monaco et al. 2018). MCS has the potential, theoretical advance of providing different degrees of haemodynamic and respiratory support (up to full cardiorespiratory support with venoarterial extracorporeal membrane oxygenation) without the potential side effects of vasoactives. In addition, the recently developed concept of mechanical unloading as new paradigm to improve outcome in heart failure and cardiogenic shock is gaining increasing popularity (Burkhoff et al. 2015; Uriel et al. 2018; Baldetti et al. 2021).
However, MCS devices are still associated with high costs, need for expertise, and potential complications themselves (Zangrillo et al. 2013) that requires careful weighing of benefit and risks in each single case (Combes et al. 2020; Rihal et al. 2015; Atkinson et al. 2016). Nevertheless, pilot studies in acute heart failure and cardiogenic shock comparing pharmacological versus mechanical support have been performed and showed controversial results, with some favouring MCS (den Uil et al. 2019; Lackermair et al. 2021), while others showed no additional benefit with immediate as compared with rescue initiation of MCS (Ostadal et al. 2023). In general, mechanical circulatory support should be considered early in case of dependency on high-dose inotropes/vasopressor {especially with vasoactive-inotropic score [VIS] (Belletti et al. 2021) >20}. In the future, with increasing clinical experience and technological advances, MCS use is likely to expand, and further trials comparing mechanical versus pharmacological support are ongoing (Banning et al. 2021; Udesen et al. 2019).
In recent years, the concept of metabolic resuscitation for patients with cardiovascular failure became increasingly popular. Metabolic resuscitation includes a combination of steroids and vitamins (vitamin C and vitamin B1) and a large number of RCTs have been performed to test these molecules alone or in various combination (Moskowitz et al. 2018; Fujii et al. 2022). After promising initial results, current evidence collectively suggest that metabolic resuscitation does not provide additional survival benefit (Fujii et al. 2022). Nevertheless, the latest Surviving Sepsis Guidelines (Evans et al. 2021) suggest the use of steroids in septic shock patients since they reduce vasopressor therapy duration and length of ICU stay without increasing adverse events (Fujii et al. 2022).
While haemodynamic management historically focused on so-called microcirculation and major haemodynamic parameters (such as MAP and CI), the role of microcirculatory dysfunction in organ dysfunction and failure in critical illness is being increasingly recognised and investigated (Østergaard et al. 2015; Ince et al. 2018). Future research should focus on the different effect of vasoactive medications on microcirculation and tissue perfusion independently of traditional haemodynamic parameters. However, a systematic review found there is no convincing evidence that any vasoactive agent can lead to improved microvascular flow, although available studies are characterised by high heterogeneity in terms of microcirculation assessment and high risk of bias (Potter et al. 2019).
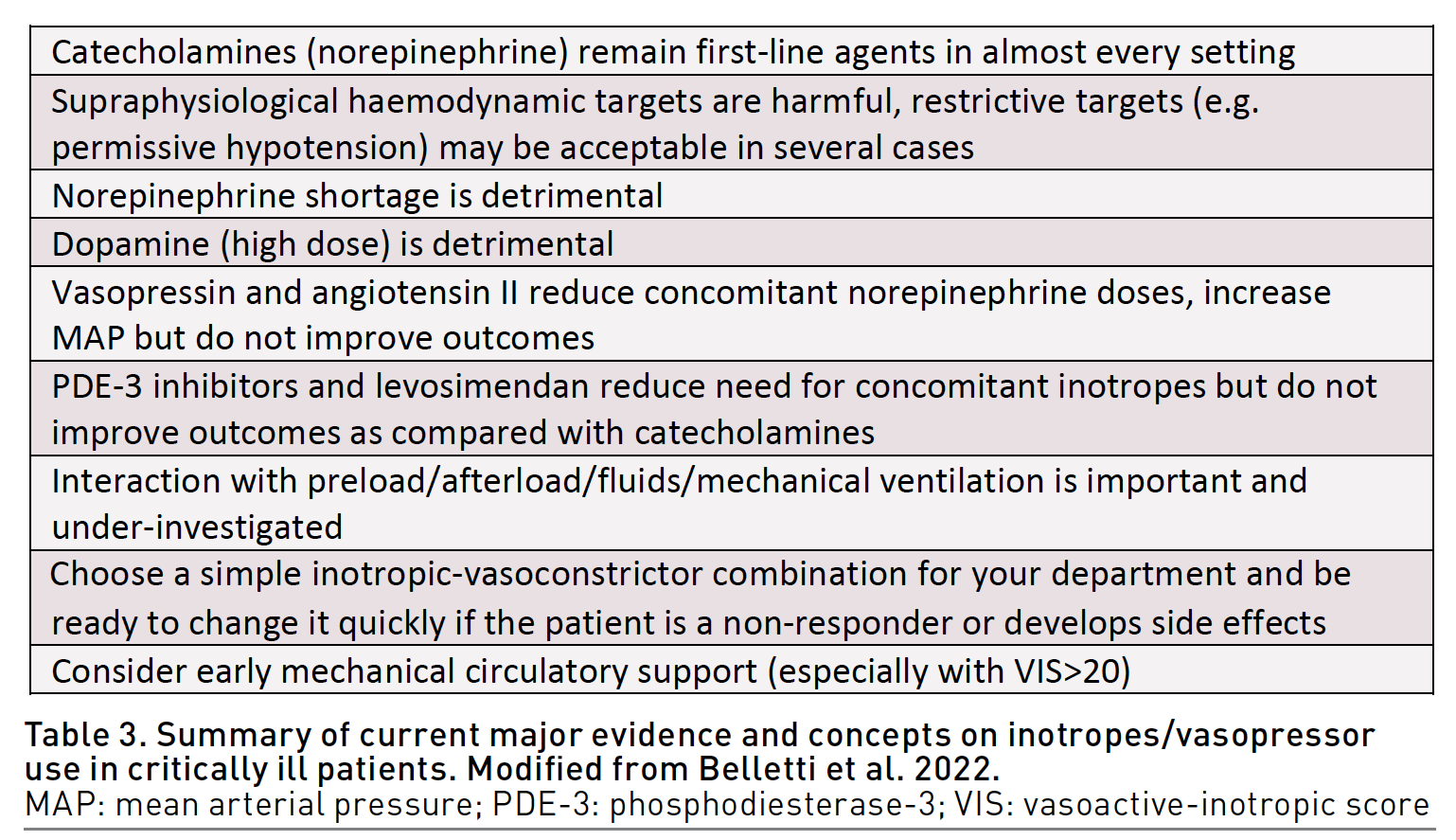
Finally, a concept of broad-spectrum vasopressors has been recently introduced (Chawla et al. 2019). Some experts suggest a combination use of different vasopressors with different mechanism of action (e.g. norepinephrine, vasopressin and angiotensin II) to reduce the dose of each drug, limit side effects, and individualise vasopressor therapy, in similar way to broad-spectrum antibiotic therapy. Whether this concept will translate into improved outcomes remains to be determined. Table 3 provides a final take-home message on inotropes and vasopressors use in critical care.
Conclusions
Inotropes and vasopressors may have relevant side effects that need to be known and acknowledged, and incorrect prescription of inotropes administration can increase morbidity and mortality. The choice of molecule or combination of molecules does not seem to influence mortality as long as comparable haemodynamic parameters are obtained. Clinicians should choose the drug or combination of drugs they are most familiar with.
Future studies should focus on identification of optimal haemodynamic targets, investigate interaction between vasoactives, fluids, pre-load and afterload, optimal timing of vasoactive initiations, and the role of MCS.
Conflict of Interest
None.
References:
Abraham WT, Adams KF, Fonarow GC et al. (2005) In-Hospital Mortality in Patients With Acute Decompensated Heart Failure Requiring Intravenous Vasoactive Medications. J Am Coll Cardiol. 46(1):57-64.
Algarni KD, Maganti M, Yau TM (2011) Predictors of low cardiac output syndrome after isolated coronary artery bypass surgery: trends over 20 years. Ann Thorac Surg. 92(5):1678-1684.
Anantasit N, Boyd JH, Walley KR, Russell JA (2014) Serious adverse events associated with vasopressin and norepinephrine infusion in septic shock. Crit Care Med. 42(8):1812-1820.
Andreis DT, Singer M (2016) Catecholamines for inflammatory shock: a Jekyll-and-Hyde conundrum. Intensive Care Med. 42(9):1387-1397.
Annane D, Ouanes-Besbes L, de Backer D et al. (2018) A global perspective on vasoactive agents in shock. Intensive Care Med. 44(6):833-846.
Annane D, Vignon P, Renault A et al. (2007) Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet. 370(9588):676-684.
Arrigo M, Mebazaa A (2015) Understanding the differences among inotropes. Intensive Care Med. 41(5):912-915.
Arulkumaran N, Corredor C, Hamilton MA et al. (2014) Cardiac complications associated with goal-directed therapy in high-risk surgical patients: a meta-analysis. Br J Anaesth. 112(4):648-659.
Asfar P, Meziani F, Hamel JF et al. (2014) High versus low blood-pressure target in patients with septic shock. N Engl J Med. 370(17):1583-1593.
Atkinson TM, Ohman EM, O’Neill WW et al. (2016) A Practical Approach to Mechanical Circulatory Support in Patients Undergoing Percutaneous Coronary Intervention: An Interventional Perspective. JACC Cardiovasc Interv. 9(9):871-883.
Avni T, Lador A, Lev S et al. (2015) Vasopressors for the Treatment of Septic Shock: Systematic Review and Meta-Analysis. PLoS One. 10(8):e0129305.
Baldetti L, Pagnesi M, Gramegna M et al. (2021) Intra-Aortic Balloon Pumping in Acute Decompensated Heart Failure With Hypoperfusion: From Pathophysiology to Clinical Practice. Circ Hear Fail. 14(11):e008527.
Bangash MN, Kong ML, Pearse RM (2012) Use of inotropes and vasopressor agents in critically ill patients. Br J Pharmacol. 165(7):2015-2033.
Banning AS, Adriaenssens T, Berry C et al. (2021) Veno-arterial extracorporeal membrane oxygenation (ECMO) in patients with cardiogenic shock: rationale and design of the randomised, multicentre, open-label EURO SHOCK trial. EuroIntervention. 16(15):E1227-E1236.
Baran DA, Grines CL, Bailey S et al. (2019) SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter Cardiovasc Interv. 94(1):29-37.
Bauer SR, Sacha GL, Lam SW (2018) Safe Use of Vasopressin and Angiotensin II for Patients with Circulatory Shock. Pharmacotherapy. 38(8):851-861.
Belletti A, Azzolini ML, Baldetti L et al. (2022) Inotropes and Vasopressors Use in Critical Care and Perioperative Medicine: Evidence-Based Approach (Review). Gen Reanimatol. 18(5):60-77.
Belletti A, Landoni G, Lomivorotov VV et al. (2020) Adrenergic Downregulation in Critical Care: Molecular Mechanisms and Therapeutic Evidence. J Cardiothorac Vasc Anesth. 34(4):1023-1041.
Belletti A, Castro ML, Silvetti S et al. (2015) The effect of inotropes and vasopressors on mortality: A meta-analysis of randomized clinical trials. Br J Anaesth. 115(5):656-675.
Belletti A, Nagy A, Sartorelli M et al. (2020) Effect of continuous epinephrine infusion on survival in critically ill patients: A meta-analysis of randomized trials. Crit Care Med. 398-405.
Belletti A, Benedetto U, Putzu A et al. (2018) Vasopressors during cardiopulmonary resuscitation. A network meta-analysis of randomized trials. Crit Care Med. 46(5):e443-e451.
Belletti A, Musu M, Silvetti S et al. (2015) Non-adrenergic vasopressors in patients with or at risk for vasodilatory shock. A systematic review and meta-analysis of randomized trials. PLoS One. 10(11):e0142605.
Belletti A, Lerose CC, Zangrillo A, Landoni G (2021) Vasoactive-Inotropic Score: Evolution, Clinical Utility, and Pitfalls. J Cardiothorac Vasc Anesth. 35(10):3067-3077.
Bignami E, Belletti A, Moliterni P et al. (2016) Clinical practice in perioperative monitoring in adult cardiac surgery: is there a standard of care? Results from a national survey. J Clin Monit Comput. 30(3):347-365.
Bitton E, Zimmerman S, Azevedo LCP et al. (2022) An international survey of adherence to Surviving Sepsis Campaign Guidelines 2016 regarding fluid resuscitation and vasopressors in the initial management of septic shock. J Crit Care. 68:144-154.
Bréchot N, Hajage D, Kimmoun A et al. (2020) Venoarterial extracorporeal membrane oxygenation to rescue sepsis-induced cardiogenic shock: a retrospective, multicentre, international cohort study. Lancet. 396(10250):545-552.
Brienza N, Biancofiore G, Cavaliere F et al. (2019) Clinical guidelines for perioperative hemodynamic management of non-cardiac surgical adult patients. Minerva Anestesiol. 85(12):1315-1333.
Burkhoff D, Sayer G, Doshi D, Uriel N (2015) Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol. 66(23):2663-2674.
Caruba T, Hourton D, Sabatier B et al. (2016) Rationale and design of the multicenter randomized trial investigating the effects of levosimendan pretreatment in patients with low ejection fraction (≤40 %) undergoing CABG with cardiopulmonary bypass (LICORN study). J Cardiothorac Surg. 11(1).
Cecconi M, Corredor C, Arulkumaran N et al. (2013) Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 17(2):209
Chawla LS, Ostermann M, Forni L, Tidmarsh GF (2019) Broad spectrum vasopressors: a new approach to the initial management of septic shock? Crit Care. 23(1):124.
Chioncel O, Parissis J, Mebazaa A et al. (2020) Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 22(8):1315-1341.
Cholley B, Caruba T, Grosjean S et al. (2017) Effect of Levosimendan on Low Cardiac Output Syndrome in Patients With Low Ejection Fraction Undergoing Coronary Artery Bypass Grafting With Cardiopulmonary Bypass: The LICORN Randomized Clinical Trial. JAMA. 318(6):548-556.
Cohn JN, Goldstein SO, Greenberg BH et al. (1998) A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. N Engl J Med. 339(25):1810-1816.
Combes A, Price S, Slutsky AS, Brodie D (2020) Temporary circulatory support for cardiogenic shock. Lancet. 396(10245):199-212.
Costanzo MR, Johannes RS, Pine M et al. (2007) The safety of intravenous diuretics alone versus diuretics plus parenteral vasoactive therapies in hospitalized patients with acutely decompensated heart failure: A propensity score and instrumental variable analysis using the Acutely Decompensated Heart Failure National Registry (ADHERE) database. Am Heart J.
Cuffe MS, Califf RM, Adams KF et al. (2002) Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 287(12):1541-1547.
Cuffe MS, Califf RM, Adams KF et al. (2000) Rationale and design of the OPTIME CHF trial: outcomes of a prospective trial of intravenous milrinone for exacerbations of chronic heart failure. Am Heart J. 139(1 Pt 1):15-22.
De Backer D, Biston P, Devriendt J et al. (2010) Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 362(9):779-789.
De Backer D, Aldecoa C, Njimi H, Vincent JL (2012) Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis. Crit Care Med. 40(3):725-730.
den Uil CA, van Mieghem NM, Bastos MB et al. (2019) Primary intra-aortic balloon support versus inotropes for decompensated heart failure and low output: a randomised trial. EuroIntervention. 15(7):586-593.
DeWitt ES, Black KJ, Thiagarajan RR et al. (2016) Effects of commonly used inotropes on myocardial function and oxygen consumption under constant ventricular loading conditions. J Appl Physiol. 121(1):7-14.
Dünser MW, Hasibeder WR (2009) Sympathetic overstimulation during critical illness: Adverse effects of adrenergic stress. J Intensive Care Med. 24(5):293-316.
Elbouhy MA, Soliman M, Gaber A et al. (2019) Early Use of Norepinephrine Improves Survival in Septic Shock: Earlier than Early. Arch Med Res. 50(6):325-332.
Evans L, Rhodes A, Alhazzani W et al. (2021) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 49(11):e1063-e1143.
Felker GM, Benza RL, Chandler AB et al. (2003) Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 41(6):997-1003.
Fellahi JL, Fischer MO, Daccache G et al. (2013) Positive inotropic agents in myocardial ischemia-reperfusion injury: a benefit/risk analysis. Anesthesiology. 118(6):1460-1465.
Fellahi JL, Parienti JJ, Hanouz JL et al. (2008) Perioperative use of dobutamine in cardiac surgery and adverse cardiac outcome: propensity-adjusted analyses. Anesthesiology. 108(6):979-987.
Feneck RO, Sherry KM, Withington PS, Oduro-Dominah A (2001) Comparison of the hemodynamic effects of milrinone with dobutamine in patients after cardiac surgery. J Cardiothorac Vasc Anesth. 15(3):306-315.
Francis GS, Bartos JA, Adatya S (2014) Inotropes. J Am Coll Cardiol. 63(20):2069-2078.
Freestone PP, Hirst RA, Sandrini SM et al. (2012) Pseudomonas aeruginosa-catecholamine inotrope interactions: a contributory factor in the development of ventilator-associated pneumonia? Chest. 142(5):1200-1210.
Fujii T, Salanti G, Belletti A et al. (2022) Effect of adjunctive vitamin C, glucocorticoids, and vitamin B1 on longer-term mortality in adults with sepsis or septic shock: a systematic review and a component network meta-analysis. Intensive Care Med. 48(1):16-24.
Funk DJ, Jacobsohn E, Kumar A (2013a) The role of venous return in critical illness and shock-part I: physiology. Crit Care Med. 41(1):255-262.
Funk DJ, Jacobsohn E, Kumar A (2013b) Role of the venous return in critical illness and shock: part II-shock and mechanical ventilation. Crit Care Med. 41(2):573-579.
Gattinoni L, Brazzi L, Pelosi P et al. (1995) A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 333(16):1025-1032.
Gillies M, Bellomo R, Doolan L, Buxton B (2005) Bench-to-bedside review: Inotropic drug therapy after adult cardiac surgery -- a systematic literature review. Crit Care. 9(3):266-279.
Giglio M, Biancofiore G, Corriero A et al. (2021) Perioperative goal-directed therapy and postoperative complications in different kind of surgical procedures: an updated meta-analysis. J Anesth Analg Crit Care. 1(1):16.
Gordon AC, Mason AJ, Thirunavukkarasu N et al. (2016) Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA. 316(5):509-518.
Gordon AC, Russell JA, Walley KR et al. (2010) The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med. 36(1):83-91.
Gordon AC, Mason AJ, Perkins GD et al. (2014) Protocol for a randomised controlled trial of VAsopressin versus Noradrenaline as Initial therapy in Septic sHock (VANISH). BMJ Open. 4(7):e005866.
Gordon AC, Perkins GD, Singer M et al. (2016) Levosimendan for the Prevention of Acute Organ Dysfunction in Sepsis. N Engl J Med. 375(17):1638-1648.
Hajjar LA, Vincent JL, Barbosa Gomes Galas FR et al. (2017) Vasopressin versus Norepinephrine in Patients with Vasoplegic Shock after Cardiac Surgery: The VANCS Randomized Controlled Trial. Anesthesiology. 126(1):85-93.
Hamilton MA, Cecconi M, Rhodes A (2011) A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 112(6):1392-1402.
Hamzaoui O, Jozwiak M, Geffriaud T et al. (2018) Norepinephrine exerts an inotropic effect during the early phase of human septic shock. Br J Anaesth. 120(3):517-524.
Hayes MA, Timmins AC, Yau E et al. (1994) Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 330(24):1717-1722.
Hernández G, Ospina-Tascón GA, Damiani LP et al. (2019) Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients With Septic Shock. JAMA. 321(7):654.
Ince C, Boerma EC, Cecconi M et al. (2018) Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 44(3):281-299.
Jentzer JC, Coons JC, Link CB, Schmidhofer M (2015) Pharmacotherapy Update on the Use of Vasopressors and Inotropes in the Intensive Care Unit. J Cardiovasc Pharmacol Ther. 20(3):249-260.
Jessen MK, Vallentin MF, Holmberg MJ et al. (2022) Goal-directed haemodynamic therapy during general anaesthesia for noncardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 128(3):416-433.
Kalogeropoulos AP, Marti CN, Georgiopoulou VV, Butler J (2014) Inotrope use and outcomes among patients hospitalized for heart failure: impact of systolic blood pressure, cardiac index, and etiology. J Card Fail. 20(8):593-601.
Kastrup M, Markewitz A, Spies C et al. (2007) Current practice of hemodynamic monitoring and vasopressor and inotropic therapy in post‐operative cardiac surgery patients in Germany: results from a postal survey. Acta Anaesthesiol Scand. 51(3):347-358.
Khanna A, English SW, Wang XS et al. (2017) Angiotensin II for the Treatment of Vasodilatory Shock. N Engl J Med. 377(5):419-430.
Kochkin AA, Likhvantsev VV, Kadantseva КК (2021) Two-component vasopressor therapy for septic shock. Messenger Anesthesiol Resusc. 18(1):57-64.
Lackermair K, Brunner S, Orban M et al. (2021) Outcome of patients treated with extracorporeal life support in cardiogenic shock complicating acute myocardial infarction: 1-year result from the ECLS-Shock study. Clin Res Cardiol. 110(9):1412-1420.
Lamontagne F, Richards-Belle A, Thomas K et al. (2020) Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients With Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA. 323(10):938-949.
Landoni G, Lomivorotov VV, Alvaro G et al. (2017) Levosimendan for Hemodynamic Support after Cardiac Surgery. N Engl J Med. 376(21).
Leone M, Goyer I, Levy B et al. (2022) Dose of norepinephrine: the devil is in the details. Intensive Care Med. 48(5):638-640.
Léopold V, Gayat E, Pirracchio R et al. (2018) Epinephrine and short-term survival in cardiogenic shock: an individual data meta-analysis of 2583 patients. Intensive Care Med. 44(6):847-856.
Levy B, Clere-Jehl R, Legras A et al. (2018) Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 72(2):173-182.
Liu ZM, Chen J, Kou Q et al. (2018) Terlipressin versus norepinephrine as infusion in patients with septic shock: a multicentre, randomised, double-blinded trial. Intensive Care Med. 44(11):1816-1825.
Lomivorotov V V., Efremov SM, Kirov MY et al. (2017) Low-Cardiac-Output Syndrome After Cardiac Surgery. J Cardiothorac Vasc Anesth. 31(1):291-308.
Lowes BD, Tsvetkova T, Eichhorn EJ et al. (2001) Milrinone versus dobutamine in heart failure subjects treated chronically with carvedilol. Int J Cardiol. 81(2-3):141-149.
Maack C, Eschenhagen T, Hamdani N et al. (2019) Treatments targeting inotropy. Eur Heart J. 40(44):3626-3644.
Maganti M, Badiwala M, Sheikh A et al. (2010) Predictors of low cardiac output syndrome after isolated mitral valve surgery. J Thorac Cardiovasc Surg. 140(4):790-796.
Maganti MD, Rao V, Borger MA et al. (2005) Predictors of low cardiac output syndrome after isolated aortic valve surgery. Circulation. 112(9 Suppl).
Mathew R, Di Santo P, Jung RG et al. (2021) Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. N Engl J Med. 385(6):516-525.
McDonagh TA, Metra M, Adamo M et al. (2021) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 42(36):3599-3726.
Mebazaa A, Pitsis AA, Rudiger A et al. (2010) Clinical review: practical recommendations on the management of perioperative heart failure in cardiac surgery. Crit Care. 14(2):201.
Mebazaa A, Tolppanen H, Mueller C et al. (2016) Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 42(2):147-163.
Mebazaa A, Combes A, van Diepen S et al. (2018) Management of cardiogenic shock complicating myocardial infarction. Intensive Care Med. 44(6):760-773.
Mebazaa A, Parissis J, Porcher R et al. (2011) Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 37(2):290-301.
Mebazaa A, Nieminen MS, Packer M et al. (2007) Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 297(17):1883-1891.
Mebazaa A, Nieminen MS, Filippatos GS et al. (2009) Levosimendan vs. dobutamine: outcomes for acute heart failure patients on beta-blockers in SURVIVE. Eur J Heart Fail. 11(3):304-311.
Mehta RH, Leimberger JD, van Diepen S et al. (2017) Levosimendan in Patients with Left Ventricular Dysfunction Undergoing Cardiac Surgery. N Engl J Med. 376(21):2032-2042.
Mehta RH, Van Diepen S, Meza J et al. (2016) Levosimendan in patients with left ventricular systolic dysfunction undergoing cardiac surgery on cardiopulmonary bypass: Rationale and study design of the Levosimendan in Patients with Left Ventricular Systolic Dysfunction Undergoing Cardiac Surgery Requiring Cardiopulmonary Bypass (LEVO-CTS) trial. Am Heart J. 182:62-71.
Metra M, Nodari S, D’Aloia A et al. (2002) Beta-blocker therapy influences the hemodynamic response to inotropic agents in patients with heart failure: a randomized comparison of dobutamine and enoximone before and after chronic treatment with metoprolol or carvedilol. J Am Coll Cardiol. 40(7):1248-1258.
Møller MH, Granholm A, Junttila E et al. (2018) Scandinavian SSAI clinical practice guideline on choice of inotropic agent for patients with acute circulatory failure. Acta Anaesthesiol Scand. 62(4):420-450.
Møller MH, Claudius C, Junttila E et al. (2016) Scandinavian SSAI clinical practice guideline on choice of first-line vasopressor for patients with acute circulatory failure. Acta Anaesthesiol Scand. 60(10):1347-1366.
Monaco F, Belletti A, Bove T et al. (2018) Extracorporeal Membrane Oxygenation: Beyond Cardiac Surgery and Intensive Care Unit: Unconventional Uses and Future Perspectives. J Cardiothorac Vasc Anesth. 32(4):1955-1970.
Mongardon N, de Roux Q, Leone M, Guerci P (2023) Norepinephrine formulation for equivalent vasopressive score. Crit Care. 27(1):62.
Mortara A, Oliva F, Metra M et al. (2014) Treatment with inotropes and related prognosis in acute heart failure: contemporary data from the Italian Network on Heart Failure (IN-HF) Outcome registry. J Heart Lung Transplant. 33(10):1056-1065.
Moskowitz A, Andersen LW, Huang DT et al. (2018) Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit Care. 22(1):283.
Myburgh JA, Higgins A, Jovanovska A et al. (2008) A comparison of epinephrine and norepinephrine in critically ill patients. Intensive Care Med. 34(12):2226-2234.
NHLBI Prevention and Early Treatment of Acute Lung Injury Clinical Trials Network (2023) Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N Engl J Med. 388(6):499-510.
Nielsen DV, Hansen MK, Johnsen SP et al. (2014) Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology. 120(5):1098-1108.
Nielsen DV, Algotsson L (2015) Outcome of inotropic therapy: is less always more? Curr Opin Anaesthesiol. 28(2):159-164.
Nieminen MS, Fruhwald S, Heunks LMA et al. (2013) Levosimendan: current data, clinical use and future development. Hear Lung Vessel. 5(4):227-245.
O’Connor CM, Gattis WA, Uretsky BF et al. (1999) Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 138(1 Pt 1):78-86.
Orme RML, Perkins GD, McAuley DF et al. (2014) An efficacy and mechanism evaluation study of Levosimendan for the Prevention of Acute oRgan Dysfunction in Sepsis (LeoPARDS): protocol for a randomized controlled trial. Trials. 15(1).
Ørstavik O, Ata SH, Riise J et al. (2014) Inhibition of phosphodiesterase-3 by levosimendan is sufficient to account for its inotropic effect in failing human heart. Br J Pharmacol. 171(23):5169-5181.
Osawa EA, Rhodes A, Landoni G et al. (2016) Effect of Perioperative Goal-Directed Hemodynamic Resuscitation Therapy on Outcomes Following Cardiac Surgery: A Randomized Clinical Trial and Systematic Review. Crit Care Med. 44(4):724-733.
Ostadal P, Rokyta R, Karasek J et al. (2023) Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock: Results of the ECMO-CS Randomized Clinical Trial. Circulation. 147(6).
Østergaard L, Granfeldt A, Secher N et al. (2015) Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiol Scand. 59(10):1246-1259.
Overgaard CB, Dzavík V (2008) Inotropes and Vasopressors: Review of Physiology and Clinical Use in Cardiovascular Disease. Circulation. 118:1047-1056.
Packer M, Carver JR, Rodeheffer RJ et al. (1991) Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 325(21):1468-1475.
Packer M, Colucci W, Fisher L et al. (2013) Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail. 1(2):103-111.
Papp Z, Édes I, Fruhwald S et al. (2012) Levosimendan: molecular mechanisms and clinical implications: consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol. 159(2):82-87.
Pearse RM, Harrison DA, MacDonald N et al. (2014) Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 311(21):2181-2190.
Permpikul C, Tongyoo S, Viarasilpa T et al. (2019) Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER). A Randomized Trial. Am J Respir Crit Care Med. 199(9):1097-1105.
Pollesello P, Parissis J, Kivikko M, Harjola VP (2016) Levosimendan meta-analyses: Is there a pattern in the effect on mortality? Int J Cardiol. 209:77-83.
Potter EK, Hodgson L, Creagh-Brown B, Forni LG (2019) Manipulating the Microcirculation in Sepsis - the Impact of Vasoactive Medications on Microcirculatory Blood Flow: A Systematic Review. Shock. 52(1):5-12.
Rihal CS, Naidu SS, Givertz MM et al. (2015) 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol. 65(19):e7-e26.
Rona G (1985) Catecholamine cardiotoxicity. J Mol Cell Cardiol. 17(4):291-306.
Rossinen J, Harjola VP, Siirila-Waris K et al. (2008) The use of more than one inotrope in acute heart failure is associated with increased mortality: a multi-centre observational study. Acute Card Care. 10(4):209-213.
Russell JA, Walley KR, Singer J et al. (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 358(9):877-887.
Russell JA, Walley KR, Gordon AC et al. (2009) Interaction of vasopressin infusion, corticosteroid treatment, and mortality of septic shock. Crit Care Med. 37(3):811-818.
Scheeren TWL, Bakker J, Kaufmann T et al. (2021) Current use of inotropes in circulatory shock. Ann Intensive Care. 11(1):21.
Schmittinger CA, Torgersen C, Luckner G et al. (2012) Adverse cardiac events during catecholamine vasopressor therapy: a prospective observational study. Intensive Care Med. 38(6):950-958.
Schoemaker WC, Appel PL, Kram HB (1988) Tissue oxygen debt as a determinant of lethal and nonlethal postoperative organ failure. Crit Care Med. 16(11):1117-1120.
Serpa Neto A, Nassar AP, Cardoso SO et al. (2012) Vasopressin and terlipressin in adult vasodilatory shock: a systematic review and meta-analysis of nine randomized controlled trials. Crit Care. 16(4):R154.
Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS (2001) Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 189(3):257-265.
Shahin J, deVarennes B, Tse CW et al. (2011) The relationship between inotrope exposure, six-hour postoperative physiological variables, hospital mortality and renal dysfunction in patients undergoing cardiac surgery. Crit Care. 15(4):R162.
Tacon CL, McCaffrey J, Delaney A (2012) Dobutamine for patients with severe heart failure: a systematic review and meta-analysis of randomised controlled trials. Intensive Care Med. 38(3):359-367.
Tarvasmäki T, Lassus J, Varpula M et al. (2016) Current real-life use of vasopressors and inotropes in cardiogenic shock - adrenaline use is associated with excess organ injury and mortality. Crit Care. 20(1):208.
Thackray S, Easthaugh J, Freemantle N, Cleland JGF (2002) The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure-a meta-regression analysis. Eur J Heart Fail. 4(4):515-529.
Thiele RH, Nemergut EC, Lynch C (2011a) The physiologic implications of isolated alpha(1) adrenergic stimulation. Anesth Analg. 113(2):284-296.
Thiele RH, Nemergut EC, Lynch C (2011b) The clinical implications of isolated alpha(1) adrenergic stimulation. Anesth Analg. 113(2):297-304.
Tumlin JA, Murugan R, Deane AM et al. (2018) Outcomes in Patients with Vasodilatory Shock and Renal Replacement Therapy Treated with Intravenous Angiotensin II. Crit Care Med. 46(6):949-957.
Udesen NJ, Møller JE, Lindholm MG et al. (2019) Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. Am Heart J. 214:60-68.
Uriel N, Sayer G, Annamalai S et al. (2018) Mechanical Unloading in Heart Failure. J Am Coll Cardiol. 72(5):569-580.
Vail E, Gershengorn HB, Hua M et al. (2017) Association Between US Norepinephrine Shortage and Mortality Among Patients With Septic Shock. JAMA. 317(14):1433-1442.
Van Diepen S, Katz JN, Albert NM et al. (2017) Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 136(16):e232-e268.
van Diepen S (2018) Norepinephrine as a First Line Inopressor in Cardiogenic Shock: Oversimplification or Best Practice? J Am Coll Cardiol. 72(2):183-186.
van Diepen S, Mehta RH, Leimberger JD et al. (2020) Levosimendan in patients with reduced left ventricular function undergoing isolated coronary or valve surgery. J Thorac Cardiovasc Surg. 159(6):2302-2309.e6.
Vasu TS, Cavallazzi R, Hirani A et al. (2012) Norepinephrine or dopamine for septic shock: systematic review of randomized clinical trials. J Intensive Care Med. 27(3):172-178.
Vincent JL, Ince C, Bakker J (2012) Clinical review: Circulatory shock--an update: a tribute to Professor Max Harry Weil. Crit Care. 16(6):239.
Williams JB, Hernandez AF, Li S et al. (2011) Postoperative Inotrope and Vasopressor Use Following CABG: Outcome Data from the CAPS-Care Study. J Card Surg. 26(6):572-578.
Wilkman E, Kaukonen KM, Pettilä V et al. (2013) Association between inotrope treatment and 90-day mortality in patients with septic shock. Acta Anaesthesiol Scand. 57(4):431-442.
Xamoterol in severe heart failure (1990) The Xamoterol in Severe Heart Failure Study Group. Lancet. 336(8706):1-6.
Zangrillo A, Lomivorotov VV, Pisano A et al. (2020) Long-term outcome of perioperative low cardiac output syndrome in cardiac surgery: 1-year results of a multicenter randomized trial. J Crit Care. 58:89-95.
Zangrillo A, Alvaro G, Pisano A et al. (2016) A randomized controlled trial of levosimendan to reduce mortality in high-risk cardiac surgery patients (CHEETAH): Rationale and design. Am Heart J. 177:66-73.
Zangrillo A, Landoni G, Biondi-Zoccai G et al. (2013) A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 15(3):172-178.










