ICU Management & Practice, Volume 18 - Issue 1, 2018
Traditionally, the concerns in the ICU were about meeting energy requirements while protein levels were rarely considered. Early work carried out in the 1920s by Cuthbertson had largely been forgotten until the 1980s.
Conditions in the ICU result in loss of muscle mass: patients
are immobile, often have minimal energy and protein delivery, and undergo
little or no resistance exercise.30-32Twenty-one days after a single blunt injury, 16% of total
body protein is lost, 67% of it from the muscle.33Resting energy expenditure (REE) increases progressively over
the first week to 40% above normal and can still be elevated after three weeks.
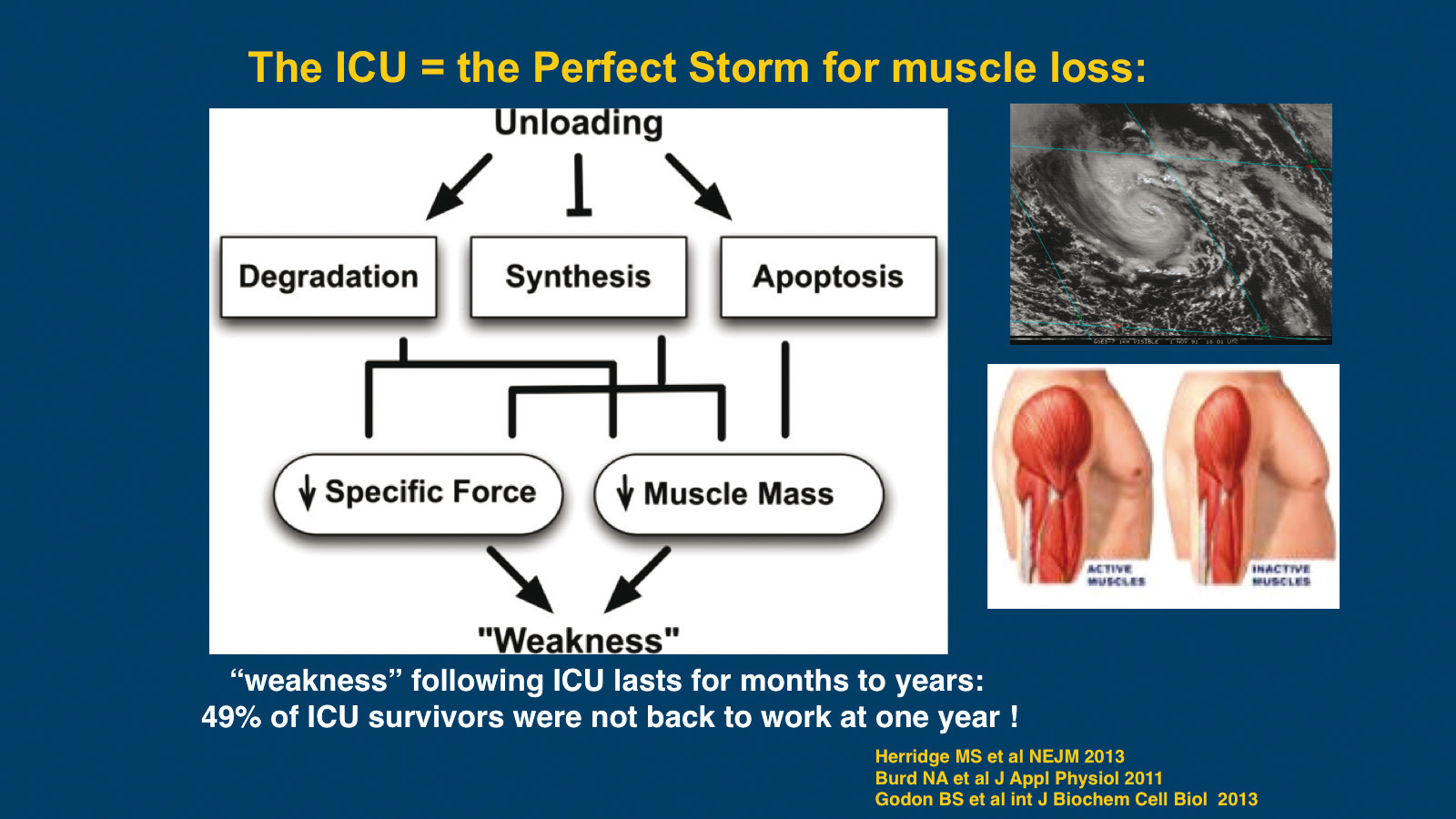
Conventional methods of analysis may not give a true picture of the rate of muscle loss. Ultrasound of the rectus femoris muscle in ICU patients showed a loss of around 10% within 10 days; but biopsies showed a thinning of muscle collagen fibres by 17.5% and when using a ratio of DNA to cellular protein, a loss of 29.5% is seen.34Significant inflammatory changes in skeletal muscle were observed despite patients being given an average of 0.7g/kg/day of protein.
Muscle loss is not confined to extremity skeletal
muscle. A study investigating muscular volumetric changes compared the
diaphragm to extremity skeletal muscle and found that there was greater loss of
the diaphragm muscle.35
A recent study investigating mechanisms of chronic
muscle wasting in elderly ICU patients found that most parameters such as
proteolysis, autophagy and inflammatory cells normalised at six months, but
satellite cells remained consistently depressed.36Satellite
cells appear to regulate the ability of muscle to recover from major loss,
therefore if these cells are compromised, there is a decreased ability to
regenerate muscle. ICU-acquired weakness and muscle wasting has a complex
aetiology but increased protein degradation, reduced protein synthesis and
often limited protein intake play a part.37
The loss of muscle mass is dramatically increased on
admission to an ICU. If inadequate protein is not supplied to these catabolic
patients muscle lost during hospitalisation may never be regained. It has been
reported that short term amino acid infusions improve protein balance and small
randomised clinical trials with parenteral nutrition show modest benefi ts in
muscle strength and fatigue.38-41
Questions still surround the optimal target for
protein. There are numerous studies supporting protein delivery in the ICU from
0.8g/kg/day up to 2.5g/kg/ day. Large observational studies of ICU patients
report most critically ill patients receive around 0.6g/kg/day of protein.
Several studies consistently support that the goal for protein delivery should
be at least 1.5g/kg/day and possibly higher.41-45
There is no consensus as yet on the upper limit. Some clinicians advocate delivery of up to 3g/kg/day (in adolescent patients), but guidelines are generally consistent in recommending an upper limit of 2.5g/kg/day.46,47There are potential issues with excess protein including azotaemia, hepatic protein synthesis and altered mental status which are more theoretical than observed.48,49
A number of studies have demonstrated that infusion of
exogenous amino acids can improve whole-body protein balance, without
increasing amino acid oxidation rates in critically ill patients.38,50,51A higher protein intake was generally associated with
an improved nitrogen balance, with dosages of 2g/kg/day being more successful
than lower intakes.52
There is also concern that protein delivery may affect
the autophagy balance. Nutrient delivery inhibits autophagy but activates
cellular protein synthesis so there is not a simple direct relationship between
feeding (or starvation) and autophagy.
Could anabolic resistance be a factor in ICU patients?
Anabolic resistance is driven by an insensitivity to the anabolic effects of
amino acids, particularly leucine. Although we do not have definitive answers
for overcoming anabolic resistance we do know that certain approaches, such as
hypercaloric PN or EN, hypocaloric feeding, use of anti-inflammatory, and
appetite stimulants do not work.
On the other hand we know that certain interventions
work consistently to protect lean body mass: protein supplementation, delivered
by pulsed bolus; early enteral feeding, which protects the gut barrier and
decreases systemic inflammation; metabolic modulation with nutrients such as
leucine, arginine, and specialised pro-resolving molecules (SPMs); glycaemic
control, resistance exercise and support for the microbiome. Other
interventions appear to work in other patient groups but have not yet been
confirmed as of benefit in the ICU.
In conclusion, there is good evidence to support
protein in the ICU is beneficial although delivery must be individualised. An
upper range of 2.5g/kg/day is considered safe. Optimal protein intake may be
different in the acute compared to the prolonged phase of illness. Due to the
heterogeneous nature of the ICU population decisions must be made on an
individual basis. Aggressive protein delivery combined with resistance exercise
may improve muscle kinetics, metabolism and regeneration. Most of our evidence
currently comes from observational trials, which may not be consistent with
RCTs and there are still many unanswered questions.
Satellite Symposium Proceeding from ESICM 2017
30th Annual Congress – Vienna,
25th September 2017
References:
31. Herridge M. N Engl J Med 2013;369:1367-9.
32. West DW. Am J Clin Nutr 2011;94:795-803.
33. Plank LD. World J Surg 2000;24:630-8.
34. Puthucheary ZA. JAMA 2013;310:1591-600.
35. Jung B. Anesthesiology 2014;120:1182-91.
36. dos Santos C. Am J Respir Crit Care Med 2016;194:821-30.
37. Schefold JC. J Cachexia Sarcopenia Muscle 2010;1:147-57.
38. Liebau F. Crit Care 2015;19:106.
39. Ferrie S. JPEN 2016;40:795-805.
40. Allingstrup MJ. Clin Nutr 2012;31:462-8.
41. Weijs PJ. Crit Care 2014;18:591.
42. Rehal MS. Crit Care 2016;20:54.
43. Alberda C. Int Care Med 2009;35:1728-37.
44. Moisey LL. Crit Care 2013;17:R206.
45. Rudrappa SS. Front Physiol 2016;August 25:361.
46. Deutz NEP. Clin Nutr 2013;32:309-13.
47. Verbruggen SCAT. Crit Care Med 2013;39:2518-25.
48. Doig GS. Intensive Care Med 2015;41:1197-208.
49. Weijs P. Crit Care 2014;18:701.
50. Kim I-Y. Am J Physiol Endocrin Met 2015;310:E73-80.
51. Berg A. Crit Care 2013;17:R158.
52. Dickerson RN. J Trauma Acute Care Surg 2012;73:549-57.






