ICU Management & Practice, Volume 21 - Issue 6, 2021
Introduction
Pulmonary Embolism (PE) is the third cardiovascular cause of death since its clinical expressions in critical patients may go unnoticed or present themselves as sudden respiratory arrest or failure, which could lead to death. The approach in the ICU is focused on timely identification, haemodynamic and respiratory support, and reperfusion therapy, either with thrombolysis or thrombectomy (Essien et al. 2019).
Epidemiology
PE affects 900,000 people per year in United States and Europe, out of which 100,000 die (Torres and Haut 2020). Its incidence has a range of 70 to 183 per 100,000 people per year (Essien et al. 2019; Ashrani and Heit 2008; Spencer et al. 2006). From 10 to 30% of these patients die within 30 days and 25% are expressed as sudden death (White 2003). The patients’ quality of life is reduced when complications such as post-thrombotic syndrome and chronic pulmonary hypertension appear. PE is seen more frequently in patients of >40 years.
Between 25 and 50% of cases do not have a clear aetiology, 20% are associated with a recent surgery and between 15 and 25% are associated with cancer. Factors associated mainly with mortality are old age, cancer, and previous heart or pulmonary diseases, which happened 30 days after a deep vein thrombosis (DVT) in 6% of the patients and after a PTE in 12% (White 2003). Patients with PTE who are not treated show a mortality of 25%, decreasing up to 1% in patients who received appropriate treatment (Essien et al. 2019). Depending on PTE seriousness, patients with normal blood pressure show a mortality of 2%, 30% in patients with right ventricular failure, and 65% in patients who go into cardiopulmonary arrest (White 2003).
PE has a mortality risk 18 times higher in comparison with patients with equal demographic conditions and those who do not have the disease (Essien 2019). The main factors of poor prognosis are presentation with syncope, shock and hypotension, right ventricular dysfunction, elevated troponins, and B-type natriuretic peptide (Vacca and Jehle 2013). Mortality is independent of the thrombus location in the pulmonary vasculitis, being higher in proximal involvement (10.7%) than in subsegmental involvement (6.5%) (Den Exter et al. 2013).
Pathophysiology
The cause of venous thromboembolism is multifactorial, commonly requiring a predisposing risk factor (e.g., thrombophilia) and/or a triggering one (e.g., surgery). A prothrombotic and proinflammatory aetiology in which coagulation factors interrelate extensively with immune cells is proposed (Khan et al. 2021). Venous thrombosis triggering mechanisms are related to Virchow's Triad, characterised by venous stasis, vascular endothelial injury, and hypercoagulability, favoured by hypoxia and constant inflammation. Tissue damage leads to the activation of von Willebrand factor, E-selectin and P-selectin receptors. Tissue factor (TF), erythrocytes, and leukocytes bind to these receptors; this binding causes activation of the direct coagulation cascade, which is exacerbated by the action of neutrophil extracellular traps (NETs). NETs activate factor XII which initiates the intrinsic coagulation pathway producing factor X and thrombin. Finally, the thrombus is formed which is composed of fibrin, NETs, platelets, and red blood cells.
Right Ventricular Failure in PE
There are anatomical and functional differences between the right and left ventricles. The right ventricle (RV) is part of the pulmonary circulation, a system of low pressures, with low vascular resistances and greater distensibility compared to the left ventricle (LV) and the systemic circulation. When a thrombotic event occurs in the pulmonary artery or arteries (Figure 1), there is a large increase in RV afterload, which is exacerbated by a vasoconstrictor effect triggered by hypoxaemia and caused by increased serotonin and thromboxane (Weinstein et al. 2021). This results in a reduction of RV stroke volume, which limits antegrade blood flow and causes dilatation of said cavity (Bryce et al. 2019). RV dilatation causes displacement of the interventricular septum in the direction of the LV causing a reduction in its SV, which in turn decreases cardiac output (CO) and is accompanied by coronary hypoperfusion. This phenomenon is known as ventricular interdependence. The increase in transmural pressure of the RV wall leads to occlusion of its corresponding coronary arteries and generation of ischaemia (Mahmood 2018). Another characteristic is tricuspid annular dilation that causes tricuspid regurgitation with reduced RV volume. In short, the sum of these events results in reduced cardiac output with reduced blood pressure, increased coronary hypoperfusion with obstructive shock and death.
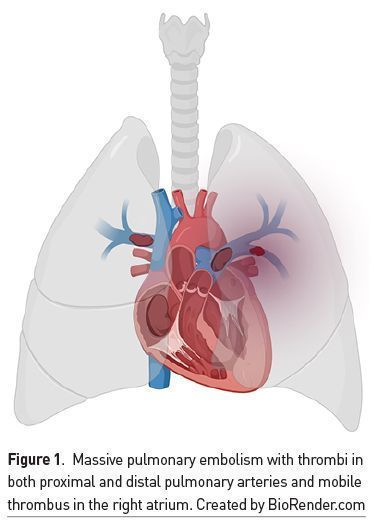
High- and Intermediate-Risk PE
A diagnosed PE that does not cause RV dysfunction is classified as mild or low-risk PE, and these patients can usually be managed on an outpatient basis. When PE is accompanied by RV dysfunction indicated by cardiac biomarkers and/or imaging studies, it is classified as intermediate-risk or submassive PE. If there is sustained hypotension (systolic blood pressure <90 mmHg for >15 minutes or a decrease of >40 mmHg) and clinical data of obstructive shock or cardiac arrest, it is categorised as high-risk or massive PE (Table 1). Massive PE occurs in 5% of patients with a mortality of 18-65% of cases; on the other hand, submassive PE occurs in 40% of patients with a mortality of 5-25% (Bryce et al. 2019). The Pulmonary Embolism Severity Index (PESI) and simplified PESI (s-PESI) (Table 2) can also be used to categorise PE into mild, intermediate, or high risk (Konstantinides et al. 2019).

Cardiac troponin elevation as a marker of cardiac necrosis is associated with higher short-term mortality and more adverse events (Becattini et al. 2007). Increased values of other cardiac biomarkers such as heart-type fatty acid-binding protein (H-FABP), N-terminal pro B-type natriuretic peptide (NT-proBNP) and brain natriuretic peptide (BNP) are associated with RV involvement.
The echocardiographic findings associated with RV dysfunction are (Figure 2):
- RV size increase in the parasternal long axis
- RV/LV relationship increase >0,9
- Flattening of the intraventricular septum in the parasternal short axis
- Absence of the inferior vena cava collapsibility
- 60/60 sign
- Mobile thrombus in the right atrium or RV
- TAPSE <16mm
- Decreased tricuspid annular peak systolic velocity <9,5 cm/s
 Evaluation of a Critical Patient With PE
Evaluation of a Critical Patient With PE

The main symptoms of patients with PE are commonly sudden onset dyspnoea and tachypnoea; tachycardia, chest pain, pelvic limb pain, fever, haemoptysis or syncope may also be experienced. Electrocardiographic changes such as sinus tachycardia, complete right bundle branch block and S wave pattern in DI, Q wave in DIII, and inverted T wave in DIII may be seen in laboratory tests although these are relatively infrequent. However, diagnosing a severely hospitalised patient under sedation and mechanical ventilation who gets complicated with PE is challenging; thus, it should be considered when patients develop sudden respiratory and haemodynamic deterioration, as tachycardia and pain may be suppressed by painkillers and sedatives. The increase in physiological dead space due to pulmonary hypoperfusion disorder may generate an increase in arterial pressure (PaCO2) and hypoxaemia, without modifications to the respiratory system compliance or resistance and, therefore, without evident modifications to the respiratory mechanics during mechanical ventilation. Electrocardiographic changes and swelling of the pelvic limbs may also be present, which may complement the diagnostic suspicion.
In a hospitalised patient with suspected or diagnosed PE, continuous monitoring of blood pressure (BP), respiratory rate (RR), heart rate (HR), peripheral partial blood oxygen saturation (SpO2), consciousness and tissue perfusion data such as capillary filling, skin colouration and temperature and uresis is recommended, intentionally looking for clinical data of shock due to haemodynamic instability. Bedside ultrasound is recommended to detect: 1) RV dysfunction data, 2) thrombi detection in the right ventricle, and 3) thrombi detection in femoral veins proximal segment. Haemodynamic management should be established immediately and, if possible, diagnostic confirmation with pulmonary angiography or ventilation/perfusion scan after stabilisation.
Specific Management
Anticoagulation
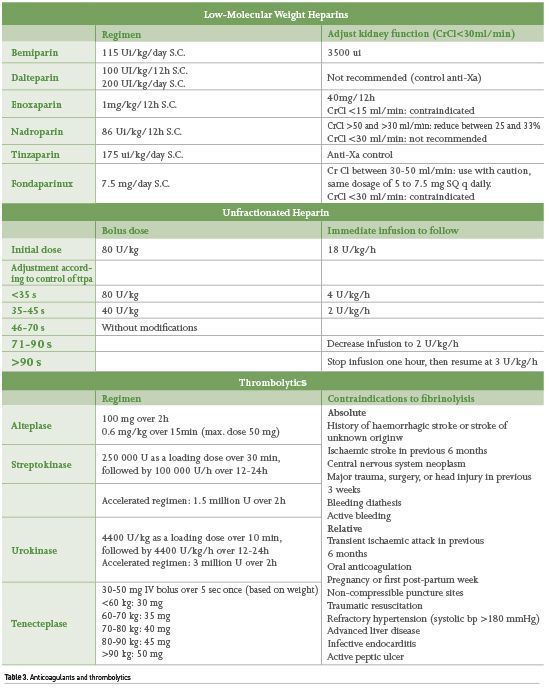
Anticoagulation therapy decreases mortality in patients with PE (Weinstein et al. 2021). Anticoagulants may be used enterally or parenterally. The group of direct oral anticoagulants (DOACs) and Vitamin K antagonists (VKAs) are included in enteral feeding; the most commonly used DOACs are rivaroxaban and apixaban, whereas VKAs include warfarin and acenocoumarin. Parenteral anticoagulants include unfractionated Heparin (UFH) and low-molecular-weight heparins (LMWHs), of which the most commonly used are enoxaparin and fondaparinux (Table 3).
DOACs are recommended over VKAs because they have fewer pharmacological interactions and have been shown to have a lower incidence of adverse effects, including clinically relevant bleeding; moreover, they do not require biochemical monitoring with serial laboratory tests which are necessary for monitoring the effectiveness of VKAs since an International Normalised Ratio (INR) of 2 to 3 must be kept. In addition, DOACs may be administered as soon as the diagnosis is confirmed and do not require prior parenteral anticoagulation, unlike VKAs. If VKAs are used, parenteral anticoagulation should be started at the same time and kept for at least 5 days and the INR value of 2 to 3 should be corroborated for at least 2 consecutive days.
As for parenteral anticoagulation, comparative studies have shown that LMWHs are associated with a decrease in thrombotic events, greater decrease in thrombus size, and less bleeding when compared to UFH; besides they are the anticoagulation treatment of choice for pregnant patients. However, some indications that might suggest using UFH are patients with haemodynamic instability and the need for surgical reperfusion therapy or other urgent major surgery (Leentjens et al. 2017).
Thrombolysis
Thrombolysis used in patients with massive PE has been successful in reducing mortality up to 50%. It is associated with faster clot dissolution but there is a risk of haemorrhage. As for thrombolysis in intermediate-risk PE, significant decrease in RV dysfunction, decreased need for escalating interventions and improved quality of life (Marti et al. 2015; Becattini et al. 2014; Kline et al. 2014), lower mortality or escalation in treatment but with a higher chance of haemorrhage (Weinstein 2021) have been reported. Thus, thrombolysis is only fully justified in case of PE with haemodynamic instability (Konstantinides et al. 2019).
Surgical Treatment
Surgical treatment of PE includes open pulmonary embolectomy or catheter-directed thrombolysis. To date, studies supporting the efficacy of surgical management are few; however, patients with high-risk PE or in cardiorespiratory arrest may benefit from this intervention. Compared to the use of a second dose of thrombolytic when there is no improvement with the initial dose, surgical management has been reported to have better results. Preoperative thrombolysis is associated with an increased risk of surgical bleeding but is not an absolute contraindication. Therefore, thrombectomy is indicated when thrombolysis is contraindicated, fails, or when cardiopulmonary resuscitation is needed; the perioperative mortality rate in these cases is <6% (Yamamoto 2018). Anticoagulation is required following surgical resolution; however, there is no consensus on when the best time is to start it and it should be indicated according to the doctor’s judgment. A regimen of anticoagulation with heparin for at least 5 days before switching to DOACs might be recommended (Konstantinides et al. 2019).
Haemodynamic and Respiratory Management in the ICU Patient With PE
Respiratory Support
Appropriate tissue oxygenation must be guaranteed in order to avoid organ failure; furthermore, it contributes to reduce pressure in pulmonary circulation and RV afterload by avoiding vasoconstriction due to a decrease in pulmonary vascular resistance mediated by hypoxaemia (Lyhne 2021). Oxygen therapy also contributes to alleviate dyspnoea and decrease work of breathing and respiratory rate. To increase the fraction of inspired oxygen (FiO2), conventional devices such as low-flow nasal cannulas, face mask or mask with oxygen reservoir with non-rebreathing valve may be used. These devices should be scaled according to the clinical situation, a goal of SpO2 from 90 to 96% or PaO2 from 60 to 90 mmHg may be recommended for most cases (Erol 2018). High-flow nasal cannula (HFNC) can deliver a FiO2 close to 1, generating a rapid increase in SpO2 and PaO2. Thermal regulation of inhaled oxygen preserves mucociliary function and contributes to device tolerance and increased flow that can reach up to 50 to 70 L/min. It could favour ventilation by increasing end-expiratory lung volume, increasing functional residual capacity, and decreasing respiratory drive and work of breathing. This benefit has been shown to be effective in retrospective studies and case reports (Aguilar-Piedras 2021; Vikas 2021; Messika 2017).
Regarding patients in whom adequate oxygenation cannot be guaranteed and who persist having tachypnoea and increased respiratory effort, noninvasive ventilation (NIV) may be used to support ventilation by releasing the load on the inspiratory muscles, conserving the negativity exerted by them and favouring venous return, unlike invasive ventilation.
In patients with high-risk PE, it is recommended to avoid intubation and invasive mechanical ventilation (IMV) when possible and to exhaust respiratory support strategies mentioned above; if these fail and there is one or more indications to start IMV, it should be carried out in the safest possible way. Using cardio-stable drugs during the rapid intubation sequence (etomidate, ketamine) may be recommended unless contraindicated. The use of propofol or midazolam for induction could decrease cardiac rate and contractility and worsen the haemodynamic status (Zamarron 2019). When starting IMV, it is recommended not to place positive end-expiratory pressure (PEEP) and to limit plateau pressure in order not to further increase intrathoracic positive pressure and afterload to the RV (Meyer 2016). There is no consensus on sedative management in this pathology but avoiding unnecessary and prolonged sedation may be associated with a better prognosis.
Fluid therapy and Diuretics
Intravenous fluids should be used with caution because the increase in RV end-diastolic pressure at the expense of preload will contribute to a decrease in LV end-diastolic volume, decrease in LV systolic volume and, thus, cardiac output, paradoxically worsening the haemodynamic status (Meyer 2016). On the other hand, the use of diuretics in normotensive patients with PE is associated with a decrease in the severity of PE without affecting renal function; theoretically, this may be due to a decrease in RV congestion and secondary venous congestion (Lim 2021).
Vasopressors, Inotropes, and Vasodilators
Norepinephrine (NE) is the vasopressor of first choice for patients in shock. In the case of PE accompanied by haemodynamic instability, NE can improve RV performance by enhancing coronary perfusion pressure (Meyer 2016). Vasopressin, methylene blue, and other vasopressors could increase BP in case of refractory shock; however, there is not enough information to issue recommendations on their use in this pathology. Dobutamine is an inodilator that can be used to improve cardiac contractility in cardiogenic shock and PE; its use can be considered under BP strict monitoring because using it could cause a decrease in BP. The combination of both drugs could also be considered if the doctor deems it appropriate and only under dynamic monitoring that may include transthoracic or transesophageal ultrasound. Inhaled nitric oxide has been shown to improve pulmonary function, but without achieving a benefit in mortality numbers.
Extracorporeal Membrane Oxygenation
Extracorporeal membrane oxygenation (ECMO) may be considered in critically ill patients with PE. Successful cases have been reported when it has been used in an arteriovenous modality in patients with high-risk PE or cardiac arrest, in order to provide cardiac and respiratory support; however, studies on this subject are few as they require a trained team and there is no consensus on when to initiate therapy (Murray 2021).
The summary of specific management and multiorgan support is presented in Figure 3.

Recurrence Prevention
Recurrent PE occurs in 7 to 30% of cases over 10 years, being more frequent in the first 12 months despite the use of oral anticoagulation and is more frequent in patients with cancer. The risk factors associated with recurrence are age, body mass index (BMI), male gender, active cancer, immobility of lower extremities, lupus anticoagulant, antiphospholipid antibodies, and protein S, C and antithrombin deficiencies (Heit et al. 2002).
Duration of Anticoagulation
Therapeutic anticoagulation should suffice with only 3 months if a provoked or transient risk factor has been identified (e.g. surgery, trauma, prolonged immobilisation, etc). The recurrence rate in these patients is 3%; however, in those patients at high risk of recurrence (e.g., active cancer, prolonged immobilisation), anticoagulation should be extended indefinitely and monitored for new thrombotic events, which can occur in up to 8% of cases. active cancer, prolonged immobilisation) should extend anticoagulation indefinitely and monitor for new thrombotic events, which can occur in up to 8% of cases, in addition to complications of anticoagulants such as haemorrhage (Konstantinides et al. 2019).
Inferior Vena Cava Filter
When there is a contraindication to pharmacological anticoagulation, an inferior vena cava filter may be indicated. This strategy has been associated with lower mortality despite associated complications such as an increased possibility of thrombotic events (like filter thrombosis), which can occur in up to 10% of cases, and DVT in up to 40% of cases. Its use in high-risk PE or combined with oral or parenteral anticoagulants is not recommended since it does not generate an added benefit (Yamamoto 2018).
Conclusion
PE is a serious disease associated with a high morbidity and mortality, which can occur in critically ill patients during hospitalisation. The recognition of this disease, its timely diagnosis and establishing an adequate treatment with multidisciplinary support can improve its prognosis.
Conflict of Interest
None.
References:
Ashrani AA, Heit JA (2008) Epidemiology of venous thromboembolism. Venous Thromboembolism Adv Dis A Clin Guid, 12(8):464–74.
Becattini C, Vedovati MC, Agnelli G (2007) Prognostic value of troponins in acute pulmonary embolism: A meta-analysis. Circulation, 116(4):427–33.
Becattini C, Beyer-westendorf J, Bluhmki E et al. (2014) Fibrinolysis for Patients with Intermediate- Risk Pulmonary Embolism, 1402–11.
Bova C, Sanchez O, Prandoni P et al. (2014) Identification of intermediate-risk patients with acute symptomatic pulmonary embolism. Eur Respir J, 44(3):694–703.
Bryce YC, Perez-johnston R, Bryce EB et al. (2019) Pathophysiology of right ventricular failure in acute pulmonary embolism and chronic thromboembolic pulmonary hypertension : a pictorial essay for the interventional radiologist. Insights Imaging, 13;10(1):18.
Den Exter PL, Van Es J, Klok FA et al. (2013) Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood, 122(7):1144–9.
Essien EO, Rali P, Mathai SC (2019) Pulmonary Embolism. Med Clin North Am, 103(3):549–64.
Goldhaber SZ, Come PC, Lee RT et al. (1993) Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet, 341(8844):507–11.
Heit JA, Mohr DN, Silverstein MD et al. (2002) Predictors of recurrence after deep vein thrombosis and pulmonary embolism: A population-based cohort study. Arch Intern Med, 160(6):761–8.
Khan F, Tritschler T, Kahn SR, Rodger MA (2021) Venous thromboembolism. Lancet, 398(10294):64–77.
Kline JA, Nordenholz KE, Courtney DM et al. (2014) Treatment of submassive pulmonary embolism with tenecteplase or placebo: Cardiopulmonary outcomes at 3 months: Multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost, 12(4):459–68.
Konstantinides S, Meyer G, Becattini C et al. (2020) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). European Heart Journal, 41(4): 543–603.
Leentjens J, Peters M, Esselink AC et al. (2017) Initial anticoagulation in patients with pulmonary embolism: thrombolysis, unfractionated heparin, LMWH, fondaparinux, or DOACs? Br J Clin Pharmacol, 83(11):2356–66.
Mahmood SS, Pinsky MR (2018) Heart-lung interactions during mechanical ventilation: the basics. Ann Transl Med, 6(18):349.
Marti C, John G, Konstantinides S et al. (2015) Systemic thrombolytic therapy for acute pulmonary embolism: A systematic review and meta-analysis. Eur Heart J, 36(10):605–14.
Meneveau N, Séronde MF, Blonde MC et al. (2006) Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest, 129(4):1043–50.
Robertson L, Jones LE (2017) Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for the initial treatment of venous thromboembolism. Cochrane Database Syst Rev, 2(2):CD001100.
Spencer FA, Emery C, Lessard D et al. (2006) The Worcester Venous Thromboembolism study: A population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med, 21(7):722–7.
Torres C, Haut ER (2020) Prevention, diagnosis, and management of venous thromboembolism in the critically ill surgical and trauma patient. Curr Opin Crit Care, 26(6):640–7.
Vacca VM, Jehle J (2013) Acute pulmonary embolism. Nursing (Lond), 43(3):25–6.
Weinstein T, Deshwal H, Brosnahan SB (2021) Advanced management of intermediate-high risk pulmonary embolism. Crit Care, 25(1).
White RH (2003) The epidemiology of venous thromboembolism. Circulation, 107(Suppl. 23):4–8.
Yamamoto T (2018) Management of patients with high-risk pulmonary embolism: a narrative review. J Intensive Care 6, 16.












