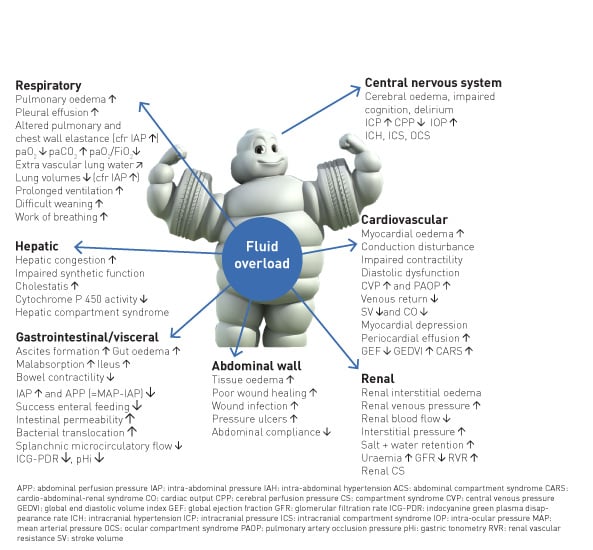
The primary goal of fluid stewardship is to optimise clinical outcomes while minimising unintended consequences of intravenous (IV) fluid administration. This article sets the stage for a conceptual framework for developing institutional programmes and guidelines to enhance fluid stewardship (especially in the ICU environment), an activity that includes appropriate selection, dosing, duration, de-escalation, and monitoring of fluid therapy. In patients with septic shock, haemodynamic stabilisation using fluids is a major challenge because clinicians are faced with many unresolved questions. It is clear that clinicians regard intravenous fluids like prescription drugs and should take into account the indications and contraindications (Perner et al. 2012; Van Regenmortel et al. 2014; Malbrain et al. 2012; Myburgh et al. 2012; Guidet et al. 2012; Annane et al. 2013), as they may affect patient-centred outcomes (Myburgh and Mythen 2013). Clinicians should also consider not only the risk of administering too little, but also too much fluid, as the deleterious consequences of fluid overload become better established (Figure 1).
1. Definitions
It is important to precisely define the various terms commonly used in the context of fluid management to describe fluid balance, fluid overload, different dynamic phases during fluid therapy, and different types of fluids; these definitions are partially based on published conceptual models (Van Regenmortel et al. 2013; Myburgh and Mythen 2013; Hoste et al. 2014; Vincent and De Backer 2013; Malbrain et al. 2014; Vincent and Pinsky 2018).
2. The four Ds of fluid therapy
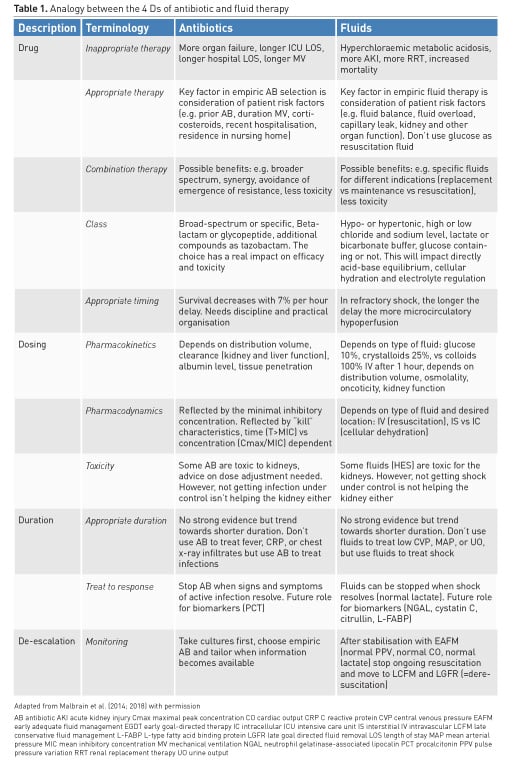
Many clinicians consider specific aspects when dealing with antibiotics: different classes, spectrum, toxicity, dose, compounds. Likewise, prescription of intravenous fluids should proceed with the same caution, taking into account the compounds, pharmacodynamic and pharmacokinetic properties of different fluids. Another way of conceptualising this is considering the "four Ds" of fluid therapy when treating patients with septic shock: drug, dosing, duration, and de-escalation (Table 1) (Malbrain et al. 2015). Fluid misuse, like antibiotic misuse, almost certainly has severe consequences for the patient.
2.1. Drug
2.1.1. Inappropriate therapy
All resuscitation, replacement and even maintenance fluids can contribute to the formation of interstitial oedema, particularly in patients with systemic inflammation associated with altered endothelial function (Myburgh and Mythen 2013). For each type of fluid, there are distinct indications: crystalloids vs colloids; synthetic vs blood-derived; balanced vs unbalanced; intravenous vs oral.
Because of their potential risk, hydroxyethyl starches are contraindicated in patients with septic shock, burns, acute or chronic kidney injury, or oliguria not responsive to fluids (Rhodes et al. 2017).
Glucose water should never be used as resuscitation fluid. Surprisingly, normal saline, which does not contain potassium, will result in higher serum potassium levels in patients with renal impairment compared to a balanced solution (lactated Ringer’s), which contains 5 mmol/L of potassium, due to concomitant metabolic acidosis secondary to a reduced strong ion difference (SID) (Khajavi et al. 2008; Langer et al. 2015). In an analogy to antibiotic treatment, where inappropriate therapy may result in more organ failure, longer ICU and hospital lengths of stay, longer duration of mechanical ventilation and higher mortality (Ibrahim et al. 2000; Hoffken and Niederman 2002), (ab)normal saline as resuscitation fluid should not be administered in large amounts, as it carries the risk of hypernatraemic hyperchloraemic metabolic acidosis, acute kidney injury (AKI) and possible RRT (renal replacement therapy), and mortality (Semler et al. 2018).
2.1.2. Appropriate therapy
Patient risk factors (prior antibiotic use, duration of mechanical ventilation, co-morbidity, hospital length of stay above 5 days, corticosteroids, recent hospitalisation, residence in nursing home,…) play important roles in the appropriate choice of empiric antibiotics (Kollef et al. 2006). Similarly, patient risk factors (fluid balance, fluid overload, capillary leak, acid-base status, co-morbidity, kidney function, organ function) also play an important role in empiric fluid therapy.
In patients with hypoalbuminaemia and septic shock, albumin may be an appropriate resuscitation fluid, especially in the late phase after the initial resuscitation (Rhodes et al. 2016; Caironi et al. 2014).
2.1.3. Combination therapy
Possible benefits of antibiotic combination therapy include: broader spectrum, synergy, avoidance of emergence of resistance, less toxicity,…(Tamma et al. 2012). Possible benefits of fluid combination therapy include: specific fluids for different indications (replacement vs maintenance vs resuscitation), and less toxicity.
2.1.4. Class
With respect to antibiotic administration, it is important to consider broad-spectrum vs specific narrower coverage classes. The choice of the antibiotic has a real impact on efficacy and toxicity. Likewise, hypotonic or hypertonic fluids, with high or low sodium or chloride level, lactate or bicarbonate buffer, glucose or not, are all equally important aspects of fluid therapy. This will have a direct impact on acid-base equilibrium, cellular hydration and electrolyte regulation.
2.1.5. Appropriate timing
Survival decreases 7% with each hour delay of antibiotic administration in patients with septic shock (Kumar et al. 2006). In refractory shock early fluid resuscitation has been proven beneficial in previous studies (Rivers et al. 2001). The longer the delay in fluid administration, the more microcirculatory hypoperfusion and subsequent organ damage (related to ischaemia reperfusion injury). Murphy et al. compared outcomes related to early adequate vs early conservative and late conservative vs late liberal fluid administration and found that the combination of early adequate and late conservative fluid management carried the best prognosis (Murphy et al. 2009).
2.2. Dosing
2.2.1. Poison
As Paracelsus nicely stated: “All things are poison, and nothing is without poison; only the dose permits something not to be poisonous.” It is the dose that makes the antibiotic poisonous, and the same holds true when it comes to fluid management in the critically ill. The risk of excessive fluid administration leading to an increase in the cumulative fluid balance has been clearly demonstrated (Malbrain et al. 2014), especially in critically ill patients with septic shock (Vincent et al. 2006) and/or acute respiratory distress syndrome (Jozwiak et al. 2013). Maintenance fluids should be used cautiously and only to cover daily needs when the patient receives no other oral or intravenous fluid intake. Their prescription should take other sources of fluids and electrolytes into account. Therefore when a patient already receives daily needs of water, glucose and electrolytes via other means (enteral or parenteral nutrition, medication solutions,…) specific maintenance fluids must be stopped.
2.2.2. Pharmacokinetics
The principle of pharmacokinetics is very well known during antibiotic administration. Pharmacokinetics (PK) describes how the body affects a drug, resulting in a particular plasma and effect site concentration (Elbers et al. 2015). PK depends on distribution volume, clearance (kidney and liver function), tissue penetration and in some situations also on albumin levels. Increased distribution volume is seen with capillary leak, hypo-oncotic states, extracorporeal circuits, surgical drains, large burns and mechanical ventilation. Pharmacokinetics of intravenous fluids depends on distribution volume, osmolality, tonicity, oncoticity and kidney function. Eventually, the half time depends on the type of fluid, but also on the patient’s condition and the clinical context. Volume kinetics is an adaptation of pharmacokinetic theory that makes it possible to analyse and simulate the distribution and elimination of intravenous fluids (Hahn 2010). The context-sensitive half-time of crystalloids and colloids may change and vary over time depending on the patient’s condition. As long as crystalloids or colloids are infused they will exert a similar volume expansion effect and their distribution and/or elimination and excretion will be slowed in cases of shock, renal failure, sedation, or general anaesthesia (Hahn 2010; 2014). This may explain why crystalloids have a much better short-term effect on the plasma volume than previously believed. Their efficiency (i.e. the plasma volume expansion divided by the infused volume) is 50-80% as long as infusion continues, and even increases to 100% when the arterial pressure has dropped. Elimination is very slow during surgery, and amounts to only 10% of that recorded in conscious volunteers.
2.2.3. Pharmacodynamics
Pharmacodynamics (PD) relates plasma concentrations to a specific effect, i.e. how the antibiotic affects the body and the bacteria. As previously described, antibiotic plasma concentrations are determined by dosing strategy, volume of distribution (Vd) and clearance (CL) (Elbers et al. 2015). Volume dynamics depends on the type of fluid used and desired location: intravascular (resuscitation), interstitial vs intracellular (cellular dehydration). Volume kinetics and dynamics depend on underlying conditions.
2.3. Duration
2.3.1. Appropriate duration
The duration of fluid therapy is equally important and the volume must be tapered when shock is resolved. However, many clinicians use certain triggers to start, but are less aware of triggers to stop fluid resuscitation, hence carrying the potential of fluid overload and all its detriments (Malbrain et al. 2014; Benes et al. 2015). As with duration of antibiotics, there is no strong evidence but a trend towards benefit from shorter duration of IV fluids (Hjortrup et al. 2016). Clinicians should not use fluids to treat low central venous pressure, mean arterial pressure, or urine output per se, but to treat shock instead. For fluids, the Frank-Starling relationship between cardiac output and cardiac preload is the equivalent of the dose effect curve for standard medications. Because of the shape of the Frank-Starling relationship, the response of cardiac output to the fluid-induced increase in cardiac preload is not constant (Monnet et al. 2017).
2.3.2. Treat to response
Clinicians should stop antibiotics when signs and symptoms of active infection resolve. Likewise, fluids should be stopped when shock is resolved (e.g. normal lactate). As with antibiotics (e.g. CRP or procalcitonin), the future role for biomarkers (e.g. neutrophil gelatinase-associated lipocalin, cystatin C, citrullin, or liver-type fatty acid binding protein) needs to be established. After the very initial fluid administration, only one half of patients with circulatory failure respond to continued intravenous fluid administration with an increase in cardiac output (Bentzer et al. 2016).
2.4. De-escalation
2.4.1. Withholding or withdrawing
The final step in fluid therapy is to consider withholding or withdrawing resuscitation fluids when they are no longer required (Malbrain et al. 2014; Benes et al. 2015; O’Connor and Prowle 2015). Along with conditioning fluid administration on the presence of fluid responsiveness, this contributes to reducing overall cumulative fluid balance. A positive cumulative fluid balance should be avoided by all means as studies have shown that fluid overload is an independent predictor for increased morbidity and mortality (Malbrain et al. 2014; Silversides et al. 2017). Eventually, active fluid removal may be indicated in some patients with global increased permeability syndrome (GIPS) and volume overload, and this is referred to as de-resuscitation. As for antibiotics (Table 1), the duration of fluid therapy must be as short as possible, and the volume (i.e. dose) must be the lowest amount effective in treating shock.
2.4.2. Monitoring
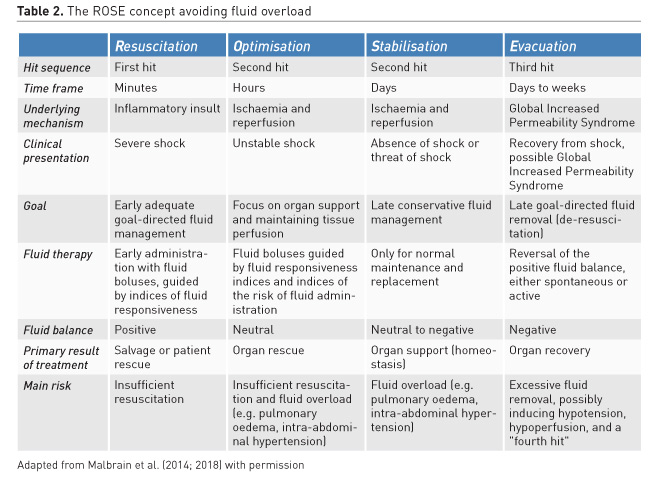
While antibiotic de-escalation may not help individual patients, it could benefit the ICU as a whole by reducing the selection pressure for resistance. Similarly, after stabilisation (normal pulse pressure variation, normal cardiac output, normal lactate) clinicians need to stop ongoing (futile) fluid resuscitation and move to de-escalation (late conservative fluid management) and de-resuscitation (late goal-directed fluid removal)(see Table 2 for explanation). However, too aggressive de-resuscitation may result in new hypoperfusion and increase in end-organ damage.
3. The four phases of fluid therapy
Recently a conceptual model of septic shock was proposed with four distinct dynamic phases of fluid therapy (Cordemans et al. 2012; Malbrain et al. 2018): Resuscitation, Optimisation, Stabilisation, and Evacuation (de-resuscitation) (R.O.S.E.) (Table 2). Specifics of the four phases are: “When to start intravenous fluids?”, “When to stop intravenous fluids?”, “When to start de-resuscitation or active fluid removal?” and finally “When to stop de-resuscitation?”
4. Fluid stewardship
4.1. Conceptual framework
The multifaceted nature of fluid stewardship will need collaboration between different disciplines such as emergency medicine, critical care, anaesthesiology, nephrology, as well as general medicine, surgery and clinical pharmacy (Malbrain et al. 2018; Dellit et al. 2007). If the primary goal of fluid stewardship is to optimise clinical outcomes while minimising unintended consequences as detailed above, then the bedside clinician needs to understand fluid physiology. The combination of effective fluid stewardship with a comprehensive fluid bundle and organ function monitoring programme should limit the deleterious effects of inappropriate fluid prescription.
The specific IV fluid need depends on the indication: whether it is intended to replace lost fluids, maintain basic metabolic needs or restore circulating volume (Malbrain et al. 2018). To determine the right type of fluid therapy for the individual patient, the clinician must choose based on the clinical exam, laboratory results and the characteristics of the available IV fluids. Though commonly prescribed, intravenous fluids are not always appropriate (Gao et al. 2015). Prescription of intravenous fluids is often done by junior doctors using either an experience-based approach, or by habit, with limited input from senior colleagues (McCrory et al. 2017; Lobo et al. 2001; Lewis et al. 2014). A multidisciplinary collaboration is an alternative approach, as has been described for antibiotic stewardship (MacDougall and Polk 2005; Paterson 2006; Doron and Davidson 2011; Schuts et al. 2016).
A comparable strategy could be used for IV fluids, by implementing ‘fluid stewardship’. There is no clear definition yet, but one preliminary example could be “a series of coordinated interventions, introduced to select the optimal fluid, dose and duration of therapy that results in the best clinical outcome, prevention of adverse events and cost reduction” (Dellit et al. 2007; Goff 2011). Similar to antibiotic stewardship, the purpose is threefold. First, the most appropriate, individualised therapy has to be chosen. It is crucial that the right fluid is prescribed in the right dose and duration and that there is a timely evaluation to start de-escalating fluid therapy (Malbrain et al. 2018). Second, early detection and prevention of inappropriate fluid administration is necessary to avoid adverse events (Bates et al. 1995). Finally, cost containment should be achieved through implementation of preventive quality improvement measures (Etchells et al. 2012).
4.2. Assessment
First, measure baseline fluid dosing, duration, costs and use patterns; second, study indications for fluid administration (resuscitation, maintenance, replacement, nutrition); and finally, identify clinician indications for prescriptions.
4.3. Goals of desirable fluid use
Goals need to be formulated and ‘appropriate’, and rational IV fluid use needs to be defined for the institution and individual patients. Empiric versus goal-directed IV fluid treatment needs to be defined for the four indications (resuscitation, maintenance, replacement, nutrition). Treatment guidelines for clinical syndromes need to be established. The appropriateness of IV fluid therapy should be assessed during the audit process. Therefore, the process of an IV fluid treatment is divided into four stages (Table 3), based on an audit framework developed by the UK National Institute for Health and Care Excellence (NICE) (Sansom and Duggleby 2014; Padhi et al. 2013).
First, the physician has to assess the patient’s IV fluid needs and decide on the right treatment (indication). Only the three major indications need to be examined thoroughly for the purpose of a clinical audit: resuscitation, maintenance and replacement or redistribution. Second, every IV fluid prescription has to be detailed in order to ensure a proper administration and a fluid management plan is available to enable continuity of care. Third, the information in the hospital’s fluid guideline or bundle is used to create different quality standards. Finally, these standards represent the necessary elements to do a full and qualitative check of appropriateness (see Table 3). If all standards are met, the therapy will be classified as appropriate for that patient.
4.4. Interventions on IV fluid prescribing
IV fluid prescribing consists of numerous elements. The day-to-day work of the core fluid stewardship members is to screen patients' medical records for appropriateness of IV fluid administration.
Further tasks may lie in the automatic review of the medical record after empiric use, fluid balance results, other laboratory data (urea and electrolytes, kidney function, albumin levels,…), and to provide advice on appropriate duration of fluid therapy. Finally, the fluid stewardship team could write an annual report to the hospital administration with calculation of clinical benefits and cost savings, if any.
4.5. Provide feedback, continuing education
The fluid stewardship team should organise a survey to test the prescriber’s knowledge about composition of fluids, indications and monitoring of fluid status. The team can provide targeted education about particular fluid composition, or one specific fluid at a time, as well as empiric versus goal-directed treatment.
4.6 Implement Fluid Stewardship
Analagous to antibiotic practice in critically ill patients, it is time to introduce fluid stewardship in the ICU, through a few simple steps. Start with a snapshot: First, check what intravenous fluids are commonly used and for what indications. Next, perform a survey of knowledge (of the nurses, doctors, pharmacists, etc). Finally, perform a clinical audit of patient records (surgical vs medical). This should be followed by a Plan-Do-Check-Act (PDCA) cycle: set up guidance (introduce fluid bundle); perform education (including tailored lectures to all junior doctors) and ward-based opportunistic teaching; and regularly re-evaluate the situation and keep increased awareness (with flyers, screen-savers, etc.).
Conclusions
There are only four major indications for fluid administration in the critically ill: resuscitation, maintenance, replacement and nutrition (enteral or parenteral).
In this review, a conceptual framework is presented looking at fluids as drugs by taking into account the four Ds (drug selection, dose, duration and de-escalation) and the four phases of fluid therapy within the ROSE concept (resuscitation, optimisation, stabilisation, evacuation). This framework will provide answers to the four basic questions surrounding fluid therapy: 1) when to start IV fluids?; 2) when to stop fluid administration?; 3) when to start fluid removal and finally 4) when to stop fluid removal? Answering these questions for each patient can provide the basis for fluid stewardship (like antibiotic stewardship) in the ICU. This fluid stewardship can be promulgated through a three-pronged attack for fluid safety: namely educating, changing prescribing habits, and increasing awareness. Good luck!
Conflict of interest
Manu Malbrain is founding President of
WSACS (The Abdominal Compartment Society, www.wsacs.org) and current Treasurer. He is also a member of the medical advisory board of Getinge (Pulsion Medical Systems) and consults for Baxter, Maltron, ConvaTec, Acelity, Spiegelberg Serenno, and Holtech Medical. He is co-founder of the
International Fluid Academy (IFA, www.fluidacademy.org).
Monty Mythen is Director of the UCL Discovery Lab. His University Chair is sponsored by Smiths Medical. He is Co-Director Duke-UCL Consortium (The Morpheus Project); a paid Consultant for Deltex Medical and Edwards Lifesciences; a Director of the Bloomsbury Innovation Group (BiG); a Director and Chair of Evidence Based Perioperative Medicine (EBPOM) Community Interest Company; Shareholder and Scientific Advisor Medical Defense Technologies LLC (Gastrostim and Entarik); Shareholder and Director Clinical Hydration Solutions ltd (Patent holder “QUENCH”); GIFTASUP guidelines – Senior Author; NICE – Expert Advisor IV Fluids – Guideline 174.
Todd Rice declares that he has no conflict of interest.
Stephanie Wuyts declares that she has no conflict of interest.