ICU Management & Practice, Volume 17 - Issue 3, 2017
In order to make haemodynamic monitoring clinically successful it seems mandatory to have a comprehensive view on the incorporation of the measured variables in a team-adapted strategy.
Over the last decades the evolution of haemodynamic monitoring in critically ill patients has not been unequivocal. On the one hand it may be argued that haemodynamic monitoring is an essential part of intensive care medicine. Analogous to mechanical ventilation, patients are specifically referred to the intensive care unit (ICU) for (better) haemodynamic monitoring. As such, haemodynamic monitoring partly defines the necessity for the existence of ICUs. This perceived importance of haemodynamic monitoring has fuelled successful progress in this field. Over the years the stage transformed from the ability to measure blood pressure and the subjective assessment of peripheral circulation (Joly and Weil 1969), to (non) invasive measurement of cardiac output (Swan and Ganz 1975), regional circulation (Fiddian-Green and Baker 1987) and even microvascular blood flow (De Backer et al. 2002). Simultaneously, static measurements were replaced by dynamic challenges (Michard and Teboul 2002) to test the physiological reserve of individual patients. And we learned to refrain from our intrinsic drive to correct the measured values under all circumstances to normal or even supra-normal (Gattinoni et al. 1995). On the other hand large series of randomised controlled trials (RCTs) failed to associate haemodynamic monitoring with improved outcome in a large variety of devices and variables (Harvey et al. 2005; ProCESS Investigators et al. 2014).
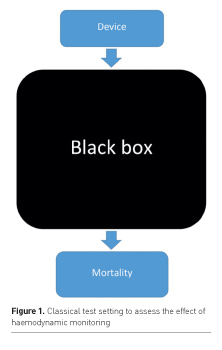
This apparent controversy may be explained by many reasons. Selection of patients, alternative strategies in the control group, inadequate signification of obtained variables, such as the classical misinterpretation of central venous pressure for preload of the right ventricle (Kumar et al. 2004), and potential adverse effects of intensified treatment may all have played a role. But one thing these RCTs all have in common is the absence of integration of the obtained variables into the diagnostic and therapeutic process. The way doctors deal with (extra) data remains a black box (Figure 1). In general a device/ variable is compared with no, or a different device/variable. Potential consequences for changes in the haemodynamic strategies are left out of the equation. In this article we aim to address a series of issues that may appear to be crucial to an effective introduction of a new haemodynamic monitoring device/ variable, but are usually not extensively addressed in the literature. Awareness of the discussed topics may help ICU decision makers to improve implementation strategies related to haemodynamic monitoring.
Pre-test likelihood
Ultimately all haemodynamic measurements will become a trigger to change or to persist in the existing haemodynamic strategy, i.e. to give fluids, maintain the dose of norepinephrine, stop dobutamine, etc. Cut-off values for such dichotomous decisions (yes/no) may be generated by static values (i.e. transfusion trigger), by trends of values over time (i.e. a decrease in blood pressure in comparison to baseline), or after specific challenges (i.e. fluid challenges). In this respect general knowledge on test results applies to haemodynamic monitoring as well. Apart from specificity and sensitivity, pre-test likelihood is of utmost importance when it comes to correct interpretation of test results. Using a test with a sensitivity of 100% and a specificity of 99.9% in a population of 10 million people for a disease with an incidence of 1 per million will inevitably lead to 10,000 false-negative test results. Improvement of the test quality is unlikely to resolve the problem, but the application of the test to the above situation is simply inadequate, creating chaos instead of solutions. Translating this to haemodynamic monitoring implies the need for a strategy to identify subgroups of patients with a considerable likelihood to have underlying haemodynamic abnormalities rather than measure a variable simply because the instrument is available. It seems key to have a predefined plan, supported by the entire ICU team, to define which patients are eligible for a specific type of haemodynamic monitoring. And it seems equally important to define which patients should not be subject to this specific type of haemodynamic monitoring.
Timing
Initiation. The moment of initiation to set up a haemodynamic monitoring device is another factor contributing to its potential success or failure. It is not uncommon to postpone the use of haemodynamic monitoring until the ICU team has run out of the usual options, most commonly the administration of fluids and norepinephrine. Although it does not seem unreasonable to try conventional therapeutic strategies before introduction of potentially dangerous invasive procedures, this strategy carries two potential risks. Haemodynamic monitoring may be helpful to diagnose underlying mechanisms for circulatory failure, for example to answer the question: Is it really cardiogenic shock, or is septic shock more likely? Knowledge about the type of shock may not only change the perspective on the haemodynamic strategy, but also on treatment of underlying causes. Delaying haemodynamic monitoring until after the initial treatment may result in non-specific data, blurring its original extremes. This phenomenon, generally referred to as regression to the mean (Morton and Torgerson 2003), is created by the fact that in critically ill patients haemodynamic monitoring only is started in those who survived the initial emergency (and treatment).
A second consequence of delaying haemodynamic monitoring is the reduction in power of its potential. In a classic RCT patients with adult respiratory distress syndrome (ARDS) were randomised for the use of a pulmonary artery catheter (PAC) (National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network et al. 2006). In short there was no difference in mortality between groups. However, the average duration to insert the PAC was almost 2 days (> 40 hours). And by that time both groups received 4.9 litres of fluids. It is hard to imagine how any form of haemodynamic monitoring could still have a therapeutic advantage under those circumstances in ARDS patients. Even if the device could provide information that would lead to the immediate cessation of additional fluid administration, the damage of fluid overload has already taken place. Correction by diuretics is not always possible and is not equal to prevention.
Since haemodynamic monitoring is not feasible under all conditions it remains reasonable to treat first that kills first. But after initial stabilisation (hours) it seems eminently reasonable to start haemodynamic monitoring as soon as possible, and not to postpone (days).
You might also like: Innovations in Monitoring: From Smartphones to Wearables
Sampling rate. Even a high-precision monitoring device may miss valuable information if the sample rate is inadequate for the situation at hand. During the construction of a subway system beneath the swampy soil of Amsterdam, engineers installed a precision monitoring instrument with mirrors and lasers, attached to the walls of historical buildings, in order to detect the slightest movement (mm!). Nevertheless in 2008 a complete block of buildings sagged suddenly, to the extent that all doors jammed and occupants had to be evacuated through the windows. Emergency constructions were needed to prevent total collapse. Was the monitoring system inadequate? No. The event measurements were performed every two hours. This example refers to the Nyquist-Shannon theorem, originating from the early days of the telephone industry, in which analogueto- digital signal conversion appeared to be a challenge. It states that in order to prevent distortion, a sample rate twice as high as the frequency of changes in the original signal is needed (Nyquist 1928). How should we translate this into haemodynamic monitoring? Since haemodynamic changes may take place within minutes, especially during interventions, monitoring on a near-continuous basis is needed to prevent unintentional drop-out of vital information. Measurements of cardiac output once per shift or even once per hour are simply inadequate. And what is true for the measurements themselves is also true for the registration of variables. Patient data management systems (PDMS) are needed to register changes in haemodynamic variables on a near-continuous basis. And they should preferably provide support in analysing trends not immediately clear to the human eye.
Human behaviour
One of the most undervalued influencing factors in the success or failure of haemodynamic monitoring is human behaviour. The vast majority of papers in this field deal with accuracy and precision. Implementation into clinical practice is generally left ‘to the discretion of the attending physician’. However, the recent FENICE study urges us to reconsider such (absence of) strategy (Cecconi et al. 2015). In centres all over Europe doctors not only defined partially wrongful endpoints of fluid resuscitation for themselves. The most shocking part of the results is the fact that in general the test result did not influence their decision to continue fluid administration. In other words patients received equal amounts of fluids, irrespective of a positive, negative or indifferent result. Under such conditions it is impossible to make a difference with haemodynamic monitoring. Recently we had similar experiences. Despite a detailed training programme the introduction of passive leg raising (PLR) in patients with septic shock did not result in a difference in median fluid balance 48 hours after ICU admission (Rameau et al. 2017). Re-evaluation with all medical and paramedical members of the ICU team revealed that compliance to test results was extremely low. During a plenary discussion representatives of all disciplines (including staff!) ‘confessed’ that they trusted their own gut feeling more than the test result. Subsequently the team decided in favour of an additional trial period, in which adherence to the test result of PLR was now key. After ‘correction’ of the behavioural issue the trial was now positive, with a significant reduction in the use of fluids.
These examples extend beyond a common implementation plan. It involves the fundamental insight that medical personnel are not only driven by reason, but by emotions and habits too. Herbert Simon introduced the Nobel prize-winning idea of heuristics (Simon 1983). He demonstrated that humans operate in what he called bounded rationality, referring to the situation where people seek solutions that are ‘good enough’ for their purposes, but could be optimised. In effect, a cognitively difficult problem is dealt with by answering a rather simpler problem, without being aware of this happening. Such an approach helps us to solve complex problems, that is to make decisions, even if we do not understand the full picture. It is a form of mental shortcut. The downside of such an approach is an unwitting rejection of new concepts and ideas, as long as we see fit to use the old ones. The unaware character of such resilience to behavioural changes with respect to healthcare professionals has profound consequences for clinical practice in general, and for the application of haemodynamic monitoring in particular. It implies that introduction of new devices and strategies is not restricted to theoretical and practical issues, such as education and training. It means that we have to monitor this process carefully, since even simple and cheap interventions, based on solid scientific principles, may not be associated with results according to our expectations (Rameau et al. 2017). Bridging this knowledge-to-care gap is one of the challenges (Cochrane et al. 2007) needed to convert a ‘simple’ haemodynamic measurement into a powerful clinical tool that has impact on morbidity and mortality of critically ill patients.
Conclusions
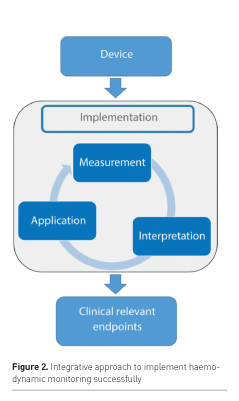
In hindsight it is not surprising that as of now there is an absence of relationship between haemodynamic monitoring and improved outcome. We have learned from the past that the success of haemodynamic monitoring depends on many aspects beyond the technical issues of accuracy and precision. Others already alluded to the idea of a chain of events needed for a positive result: correct measurement, correct interpretation and correct application (Vincent et al. 2008). This chain may become more detailed and even longer, as we better understand technical and behavioural issues of this previously black box (Figure 2). All parts of the chain need to be in place as a prerequisite for success.
Conflict of interest
Christiaan Boerma declares that he has no conflict of interest.
Abbreviations
ICU intensive care unit
PAC pulmonary artery catheter
PDMS patient data management system
PLR passive leg raising
RCT randomissed controlled trial
References:
Cochrane LJ, Olson CA, Murray S et al. (2007) Gaps between knowing and doing: understanding and assessing the barriers to optimal health care. J Contin Educ Health Prof, 27: 94-102.
De Backer D, Creteur J, Preiser JC et al. (2002) Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med, 166: 98-104.
Fiddian-Green RG, Baker S (1987) Predictive value of the stomach wall pH for complications after cardiac operations: comparison with other monitoring. Crit Care Med, 15: 153-6.
Gattinoni L, Brazzi L, Pelosi P et al. (1995) A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med, 333: 1025-32.
Harvey S, Harrison DA, Singer M et al. (2005) Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet, 366: 472-7.
Joly HR, Weil MH (1969) Temperature of the great toe as an indication of the severity of shock. Circulation, 39: 131-8.
Kumar A, Anel R, Bunnell E et al. (2004) Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med, 32: 691-9.
Michard F, Teboul JL (2002) Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest, 121: 2000-8.
Morton V, Torgerson DJ (2003) Effect of regression to the mean on decision making in health care. BMJ, 326: 1083-4.
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network et al. (2006) Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med, 354: 2213-24.
Nyquist H (1928) Certain topics in telegraph transmission theory. Transactions of the American Institute of Electrical Engineers, 47: 617-44.
ProCESS Investigators et al. (2014) A randomized trial of protocol-based care for early septic shock. N Engl J Med, 370: 1683-93.
Rameau A, de With E, Boerma EC (2017) Passive leg raise testing effectively reduces fluid administration in septic shock after correction of non-compliance to test results. Ann Intensive Care, 7: 2.
Simon HA (1983) Discovery, invention, and development: human creative thinking. Proc Natl Acad Sci U S A 80: 4569-71.
Swan HJ, Ganz W (1975) Use of balloon flotation catheters in critically ill patients. Surg Clin North Am, 55: 501-9.
Vincent JL (2008) The pulmonary artery catheter: in medio virtus. Crit Care Med, 36: 3093-6.