ICU Management & Practice, Volume 17 - Issue 1, 2017
Treating patients with multidrugresistant (MDR) pathogens is an increasing challenge for intensive care unit (ICU) physicians. In the ICU, compared to other hospital departments, severe infections are most prevalent and antimicrobial use is most abundant. Not surprisingly, antimicrobial resistance (AMR) has emerged primarily in the intensive care setting, where multiple facilitators for the development of resistance are present: high antibiotic pressure, loss of physiological barriers, and high transmission risk. There have been numerous reports on outbreaks with MDR pathogens in ICUs (French et al. 2017). In some parts of the world ICU physicians are struggling with the outlook of a “post-antibiotic era”, where there are no antibiotics available to treat common ICU infections (MacVane 2017). With no new drugs in the pipeline for MDR pathogens, implementation of an antimicrobial stewardship programme (ASP) seems a reasonable pathway to help prevent the further development of resistance (Bassetti et al. 2016).
Definition
An ASP can be thought of as a menu of interventions that is adapted and customised to fit the infrastructure and organisation of an ICU (Septimus and Owens 2011). The most important goal of an ASP is to provide safe and effective antibiotic therapy whilst safeguarding its effectiveness for future generations. This can be accomplished by reducing the total consumption of antibiotics and ensuring their appropriate usage. Interventions to reach that goal in ICU include prescription of appropriate empirical therapy, optimal timing, optimal dosing, de-escalation and discontinuation (Schuts et al. 2016).
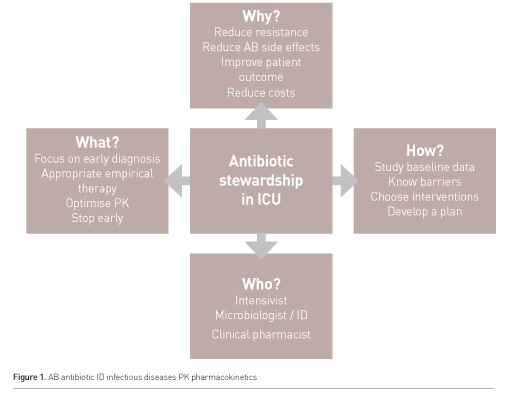
Most intensivists acknowledge the importance of antimicrobial stewardship, but have a hard time implementing an ASP in their own ICU. In this article, a stepwise approach to implementation is discussed (Figure 1).
How
to Start?

First, one needs to take control of the basics. The ICU is—more than any department in the hospital—a place where medical specialists work closely together to provide the most optimal patient care. This is especially a challenge in the treatment of patients with infections, as infectious disease physicians, clinical microbiologists and clinical pharmacists, relying on their own expertise, all advise the ICU physician on the use of antibiotics. While the ICU has become an increasingly independent place and ICU physicians have engaged in expanding clinical expertise (e.g. ultrasound, continuous renal replacement therapy), in the field of treatment of AMR infections, cooperation with other clinical specialties remains crucial. It is pivotal that intensivists take part in discussions on the hospital antibiotic formulary and help develop local guidelines for the clinical disease entities that are frequently encountered in the ICU. Preferably, an intensivist is a member of the hospital antimicrobial management team. On a day-to-day basis, the presence of an attending infectious diseases physician, clinical microbiologist or clinical pharmacist on the ICU clinical ward round may add to mutual understanding and well-deliberated treatment decisions (Rimawi et al. 2013).
Data
Some essential baseline data is needed to define if antimicrobial use in your unit is appropriate. It is important to have regularly updated information on local resistance patterns from your microbiologist. It is also essential to be aware of what the prescribing patterns are in your unit. Expressed as days of therapy (DOT) or defined daily dose (DDD)/100 patient days, it is possible to get a general feel of the (differential) of antibiotic use in your unit over time and benchmark with other comparable ICUs. Apart from these quantitative metrics it may prove useful to measure current practice closer to the patient and prescribing physician level, e.g. by assessment of the percentage of appropriate empirical therapy according to the local guidelines or the percentage of patients with DOT according to local guidelines. One could use a simple PPS (Point Prevalence Survey) or perform a small prospective audit to evaluate most of these processes (Zarb et al. 2012). These figures all together will create a picture of the current state of antimicrobial treatment in your unit and point you to the problem most in need of improvement.
Barriers
Once the largest gap is recognised, insight must be gained into the factors that influence appropriate antimicrobial prescription at the ICU and an improvement strategy should be developed based on these factors while applying social and behavioural change theories. Antimicrobial prescription is a complex process that is influenced by many factors. The appropriateness of antimicrobial use in hospitals varies between physicians, hospitals and countries, due to differences in professional background, clinical experience, knowledge, attitudes, hospital antibiotic policies, professionals’ collaboration and communication, care coordination and teamwork, care logistics and differences in sociocultural and socioeconomic factors. This renders changing hospital antimicrobial use into a challenge of formidable complexity. Given that many influencing factors play a part, the measures or strategies undertaken to improve antimicrobial use need to be equally diverse.
Even in a single ICU setting, using relatively simple methods, these challenges can be met. A well-structured group discussion focused at barriers and facilitators that influence appropriate antibiotic use can lead to surprising insights.
Interventions
A recently published Cochrane review found that most interventions are effective in increasing compliance with antibiotic policies and reducing duration of treatment. Lower use of antibiotics does not increase mortality and likely reduces length of stay. Enabling (persuasive) strategies consistently improved the effect of interventions, including those with a restrictive component (Davey et al. 2017).
Most interventions to change antibiotic use that have been studied in ICUs are effective in reducing the quantity of antibiotic use and antibiotic related costs, but the effects on clinical outcome and— importantly—on resistance levels are less outspoken (Kaki et al. 2011). The most often studied strategy in ICU is to apply restrictive interventions, such as pre-authorisation of antibiotics, e.g. by an infectious diseases specialist, a restricted antibiotic list or an automated antibiotic stop order. These are generally very effective in the short term. However, restrictive measures may wear out prescribing physicians needing to ask for permission to prescribe. More importantly, it can induce a so-called “squeeze-the-balloon effect”: by restricting one class of antibiotics, resistance will diminish for some microorganisms, but resistance to the alternative antibiotics that are used to replace the restricted ones will increase. In short, restrictive interventions are welcome in an acute outbreak setting, where there is a strong relationship between the particular antibiotic that is (over)used and the emerging resistant pathogen(s).
Many non-restrictive “persuasive” interventions such as professional education, evidence-based clinical decision support systems and guidelines, audit and feedback and reminders have also been shown to be successful in ICUs (Kaki et al. 2011; Mertz et al. 2015; Pestotnik et al. 1996).
See Also: Antibiotic Resistance in the ICU: Time to Take Things Seriously!
There is a wide variety of interventions available,
and the most difficult task is to choose the right one at the right time. These
choices are preferably made based on the insights from a thorough barrier
analysis as discussed above (Cabana et al. 1999; Flottorp et al. 2013): first
comes diagnosis, then comes treatment! If a lack of awareness or knowledge in
ICU professionals is the key problem, education could help. If, however, there
is an attitude problem, an educational strategy may prove counterproductive,
and academic detailing might work out better. Also, different aspects of
appropriate antibiotic use (start, stop or change therapy) may require very
different interventions (Schouten et al. 2005).
There is a growing body of evidence linking specific barriers to effective interventions. These can be selected and carried out. It is clear that there is no one-size-fits-all approach possible here. Rather, a more tailored approach is advocated, sometimes leading to multifaceted interventions comprising more than one type of intervention. Plan-do-study-act (PDSA) cycles can be used to target one relevant aspect of antibiotic care at the time, preferably going for the “low-hanging fruit” first.
Bedside Tools: Bundles and Biomarkers
In
ICUs, bundle approaches have often been successful: e.g. surviving sepsis
bundles, VAP bundles and CVC bundles have helped intensivists to apply the most
important aspects of a specific care setting. There are some examples of
antibiotic use bundles that cover the most relevant aspects of antibiotic use:
start, streamline and stop (Table 1). Bundles are essentially reminders, and
can be distributed as plastic flashcards, posters, a smartphone application or
even integrated within the electronic medical record.
Biomarkers can also be
used to reach antibiotic stewardship goals: the use of procalcitonin assays has
been shown to influence ICU physicians to safely shorten duration of antibiotic
therapy in ICU patients with an infection (Bouadma et al. 2010; de Jong et al.
2016).

Conclusions
In the absence of new antibiotics for
difficult-to-treat infections by MDR pathogens, antibiotic stewardship is
advocated in each ICU. ASPs aimed at combating antimicrobial resistance through
improved antibiotic use will play an increasingly important role in the ICU.
Implementation of an ASP requires a structured approach:
- First make sure the basics are taken care of: availability of information about resistance patterns and quantity and quality of antibiotic usage, engagement of a supportive team of antibiotic specialists and development of clear, locally adapted guidelines.
- Based on baseline figures, choose to target one problem at a time. Perform a thorough analysis to elucidate barriers to optimal antibiotic use using interviews or a focus group.
- Find the optimal strategy to overcome the barriers using the existing literature and common sense, involve quality of care/implementation experts to explore and implement.
- Repeat the same cycle with different targets and use PDSA to monitor progress.
Conflict of Interest
Jeroen Schouten declares that he has no conflict of interest.
Abbreviation
AMR antimicrobial resistance
ASP antimicrobial stewardship programme
DDD defined daily dose
DOT days of therapy
ICU intensive care unit
MDR multidrug resistance
PDSA plan-do-study-act
References:
Bassetti M, Poulakou G, Timsit JF (2016) Focus on antimicrobial use in the era of increasing antimicrobial resistance in ICU. Intensive Care Med, 42(6): 955-8.
Bouadma L, Luyt CE, Tubach F et al.; PRORATA trial group (2010) Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet, 375(9713): 463-74.
Cabana MD, Rand CS, Powe NR et al. (1999) Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA, 282(15): 1458-65.
Davey P, Marwick CA, Scott CL et al. (2017) Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. Feb 9(2): CD003543.
de Jong E, van Oers JA, Beishuizen A et al.
(2016) Efficacy and safety of procalcitonin guidance in reducing the duration
of antibiotic treatment in critically ill patients: a randomised, controlled,
open-label trial. Lancet Infect Dis, 16(7): 819-27.
Flottorp SA, Oxman AD, Krause J et al. (2013) A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci, 8:35.
French CE, Coope C, Conway L et al. (2017) Control of carbapenemase-producing Enterobacteriaceae outbreaks in acute settings: an evidence review. J Hosp Infect, 95(1):3-45.
Kaki R, Elligsen M, Walker S et al. (2011) Impact of antimicrobial stewardship in critical care: a systematic review. J Antimicrob Chemother, 66(6): 1223-30.
MacVane SH (2017) Antimicrobial resistance in the intensive care unit: a focus on gram-negative bacterial infections. J Intensive Care Med, 32(1):25-37.
Mertz D, Brooks A, Irfan N et al. (2015).
Antimicrobial stewardship in the intensive care setting--a review and critical
appraisal of the literature. Swiss Med Wkly, 145: w14220.
Pestotnik SL, Classen DC, Evans RS et al. (1996) Implementing antibiotic practice guidelines through computer-assisted decision support: clinical and financial outcomes. Ann Intern Med, 124(10): 884-90.
Rimawi RH, Mazer MA, Siraj DS et al. (2013) Impact of regular collaboration between infectious diseases and critical care practitioners on antimicrobial utilization and patient outcome. Crit Care Med, 41(9): 2099-107.
Schouten JA, Hulscher ME, Kullberg BJ et al. (2005) Understanding variation in quality of antibiotic use for community-acquired pneumonia: effect of patient, professional and hospital factors. J Antimicrob Chemother, 56(3): 575-82.
Schuts EC, Hulscher ME, Mouton JW et al. (2016) Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis, 16(7): 847-56.
Septimus E, Owens RC Jr (2011) Need and potential of antimicrobial stewardship in community hospitals. Clinical Infectious Diseases, 53 (suppl 1): S4-S8.
Zarb P, Coignard B, Griskeviciene J et al. National Contact Points for the ECDC pilot point prevalence survey.; Hospital Contact Points for the ECDC pilot point prevalence survey (2012)The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Euro Surveill, 17(46).







