This article describes the rise in PICU-acquired morbidities and its impact on patient outcomes. It discusses early rehabilitation strategies to improve patient outcomes in PICU.
Introduction
Critical care has traditionally been focused on early recognition of life-threatening conditions, resuscitation, and stabilisation of organ dysfunction, and ultimately improving mortality. Our ability to deliver critical care and advanced life support has continued to improve over the last two decades, and as a result, mortality rates in children admitted to Paediatric Intensive Care Units (PICUs) have fallen significantly to an all-time low of 1- 3% in developed nations (Burns et al. 2014; Hartmann et al. 2017; Namachivayam et al. 2010). Increased survival amongst critically ill children has resulted in the following consequences: Firstly, a population shift. Patients admitted to PICUs today are sicker and more complex. Critically ill children with pre-existing chronic co-morbidities have risen significantly; these patients now constitute 53-68% of the PICU population (Choong et al. 2018; Pinto et al. 2017). This group of patients has a significant impact on PICU practice and resources. They require the majority of our invasive therapies; they occupy the longest duration of stay, and they consume 81% of costs within the PICU (Briassoulis et al. 2004; Rennick and Childerhose 2015). Furthermore, they are our future patients; 35% of these children are readmitted to the hospital within 6 months following PICU discharge (Choong et al. 2018). The second consequence of improved survival amongst critically ill children is a significant rise in PICU-acquired complications (PACs) amongst survivors (Pollack et al. 2014). The incidence of PACs has risen dramatically, and now far exceeds mortality. PACs are undesirable and unintended sequelae, distinct from the admission diagnosis, and acquired during their course of a child’s PICU stay. Specifically, these include but are not restricted to iatrogenic withdrawal, delirium, and PICU-acquired weakness.
The rise of PICU-acquired complications
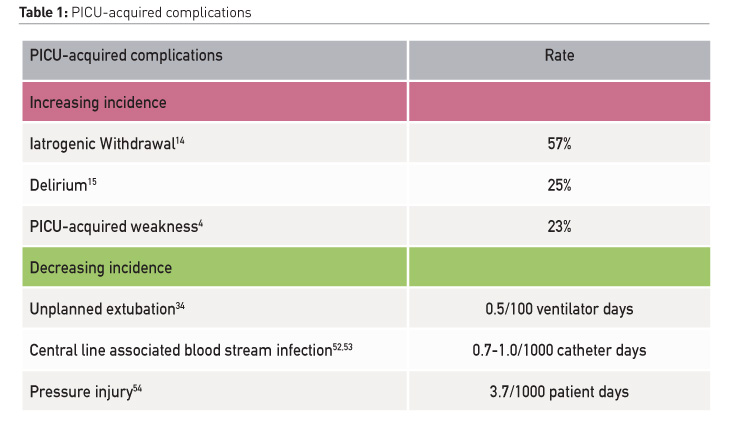
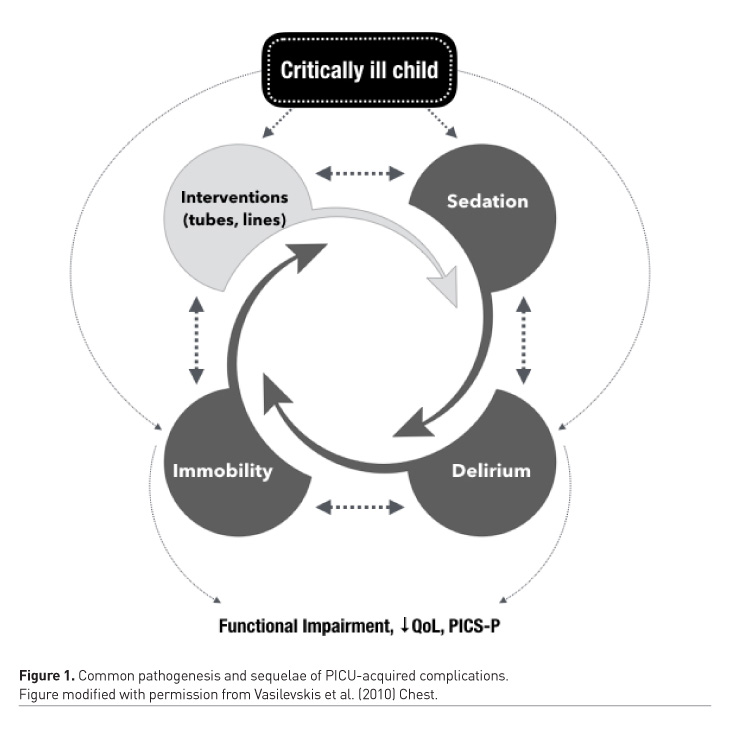
The traditional practice in many PICUs is to sedate, restrain and immobilise critically ill children as they are often considered “too sick to move” (Choong et al. 2013). Subsequently, the majority of critically ill children are excessively sedated, and prolonged bed rest is a common practice in PICUs (Choong et al. 2013; Choong et al. 2014; Garcia Guerra et al. 2016). This paradigm, along with a lack of clinician awareness of harmful sequelae (Long and Williams 2016), has led to an increase in the following specific PACs in the last 10 years: iatrogenic withdrawal syndrome has risen from 17 to 57% (Anand et al. 2010; LaRosa and Aponte-Patel 2019), delirium has increased from 10% to 25% overall and affects as many as 53% of mechanically ventilated children (Traube et al. 2017; Creten et al. 2011). PICU-acquired weakness, which less than a decade ago affected only 2%, now affects as many as 23% children (Table 1) (Choong et al. 2018; Kukreti et al. 2014). These morbidities are inter-related. Sedation depresses respiratory effort and prolongs mechanical ventilation; it hinders mobility and increases the risk of delirium and iatrogenic drug withdrawal (Silver et al. 2015; Ista et al. 2007). Immobility during critical illness causes neuromuscular atrophy and weakness (Koo et al. 2011), it exacerbates pain and agitation, further perpetuating the cycle of sedative administration. Immobility is also an independent risk factor for delirium (Vet et al. 2013; Kudchadkar et al. 2014). Sleep is commonly disrupted in critically ill patients, due to numerous factors such as a disruptive environment, invasive interventions, interruptions for nursing care, pain related to the underlying illness and pharmacological interventions (Kudchadkar et al. 2014). Sleep disruption results in delirium and insomnia, in addition to impaired immunity, catabolism, and respiratory compromise. Over-sedation, delirium, and weakness are therefore not distinct critical illness complications (Figure 1). Their pathogeneses are interrelated, and they lead to common adverse short and long-term outcomes (Vasilevskis et al. 2010). PACs are common; 61% of critically ill children develop one or more PACs (Choong et al. 2018). PACs are important to clinicians as well as patients; they are strongly associated with prolonged mechanical ventilation, longer hospitalisation, and higher mortality (Kukreti et al. 2014; Ista et al. 2007; Traube et al. 2017). The development of one or more of these PACs is associated with an increased risk of poor functional recovery, poor quality of life, persistent neurocognitive and psychological sequelae, and increased parental stress following PICU discharge (Choong et al. 2018). Collectively these constitute the post-intensive care syndrome which we now understand affects a significant proportion of paediatric survivors and their families, as it does critically ill adults (Watson et al. 2018).
Safety priorities and knowledge gaps
Despite its increasing incidence, PACs continue to be under-recognised amongst PICU clinicians. Sedation is often prioritised over awakening and mobilisation, as key safety concerns are most commonly focused on preventing unplanned extubation (da Silva et al. 2008). Clinicians are comfortable with sedating patients, but are often uncomfortable with mobilisation, and allowing children to awaken (Long and Williams 2016; Treble-Barna et al. 2019). Clinicians therefore often have conflicting attitudes towards sedation – we understand the potential side effects, yet we express the desire for more sedation for our patients (Flaigle et al. 2016). Further challenges faced by PICU clinicians is that the majority of intubated children are infants and toddlers or because of developmental disabilities, are non-verbal. Movement and wakefulness in these children are therefore often interpreted as agitation and the need to escalate sedation. Benzodiazepines and opioids are subsequently administered to facilitate sleep, but to the contrary, these medications decrease restorative sleep and increase arousal frequency, leading to further agitation and deterioration in sleep quality (Kudchadkar et al. 2014). Many PICU physicians admit not recognising nor understanding how to assess for delirium in children (Long and Williams 2016). There is a lack of awareness that critically ill children are at significant risk of delirium, and that sleep disruption is extremely common (Garcia Guerra et al. 2016; Kudchadkar et al. 2014). Subsequently, there is a paucity of routine monitoring for delirium and non-pharmacological sleep promotion in PICUs worldwide (Kudchadkar et al. 2014).
In summary, the rise in PICU-acquired complications are not only due to increasing complexity and co-morbidities amongst the critically ill paediatric population, but in large part attributable to a traditional practice paradigm of excessive sedation, prolonged immobility, knowledge gaps with respect to the risk of our current practice and subsequently, a lack of standardised strategies for the monitoring of and prevention of these sequelae.
Harm reduction and quality improvement
As the majority of critically ill children survive, mortality is no longer the most appropriate quality indicator of our care (Goodacre et al. 2015). Quality of life and function are now recognised as more meaningful and prioritised outcomes for patients and families (Merritt et al. 2018). PACs are common and harmful, but preventable, and therefore represent an opportunity for patient-centred, quality improvement in the PICU (Choong et al. 2018). As PACs impact long-term patient outcomes, reducing PACs may therefore not only improve PICU outcomes, but optimise functional recovery post-PICU discharge.
As over-sedation, withdrawal, delirium, and immobility are inter-related, single-pronged, independent interventions may not be the most effective approach to improve a common end-point of functional recovery (Craig et al. 2008). This may in part explain the lack of efficacy in previous studies targeted only at sedation or early mobilisation in isolation (Vet et al. 2016; Curley et al. 2015; Morris et al. 2016). Rather than providing different solutions to the same problem, addressing PACs collectively through a bundle of complementary quality improvement interventions enhances uptake and promotes multi-disciplinary team collaboration (Dixon-Woods et al. 2012). Promoting early rehabilitation as harm reduction emphasises the importance of reducing ICU-acquired morbidities in optimising functional outcome and quality of life in critically ill patients. Implementing ICU-based rehabilitation through an “ABCDEF” Bundle is currently a topic of much research in critical care, and has been demonstrated in adults to significantly improve symptom-related outcomes such as the duration of mechanical ventilation, coma and delirium, as well as improved system and patient-related outcomes such as survival, hospital discharge and ICU readmission rates (Pun et al. 2018). Furthermore, there appears to be a dose-response relationship between higher proportional bundle performance and improvements in these outcomes. With respect to the evidence for PICU-based rehabilitation the current evidence suggests that less is more: less sedatives, less benzodiazepines, and less immobilisation may reduce the length of hospital stay, reduce the risk of delirium, and improve adaptive functional outcomes (Fink et al. 2019; Mody et al. 2018; Simone et al. 2017; Penk et al. 2018). Minimum, effective sedation and analgesia has been shown to be safe, enables spontaneous breathing, improves sleep, reduces withdrawal and facilitates earlier mobilisation (Kudchadkar et al. 2014; Curley et al. 2015). Early mobility-based rehabilitation is feasible and safe in critically ill children (Choong et al. 2017; Cuello-Garcia et al. 2018). Implementing PICU-based early rehabilitation has been shown in preliminary studies to improve the time to and duration of mobilisation (Fink et al. 2019; Choong et al. 2017). Importantly, employing a rehabilitation bundle improves the unit culture through improved family engagement team collaboration and communication (Kawai et al. 2018; Costa et al. 2018). Whether an early rehabilitation bundle leads to improved short and longer-term patient important outcomes in critically ill children is a subject of ongoing research (Choong et al. ongoing research).
Early PICU-based rehabilitation, shifting the paradigm
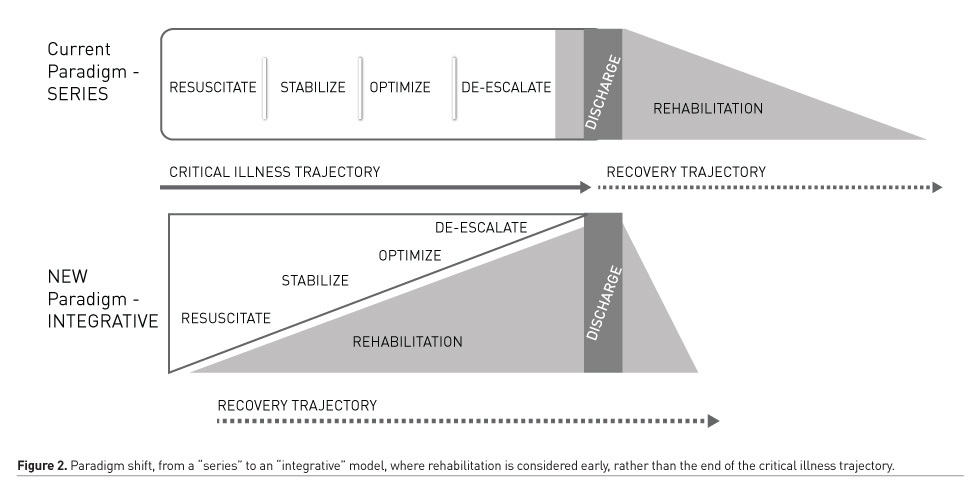
While applying an ABCDEF bundle addresses PACs collectively, implementing PICU-based rehabilitation is complex and requires significant education, team collaboration, institutional buy-in, and continuous audit and feedback mechanisms to ensure sustainability (Balas et al. 2019). No longer is the critical care clinician’s responsibility restricted to early recognition of clinical decompensation, resuscitation and improving survival, as the majority of children survive their critical illness. These patients are our future patients; we have a responsibility to our survivors to improve their survivorship, beginning within the PICU, to beyond the PICU. Creating a culture of practice begins with education and increasing awareness of PACs. Emerging paediatric-specific evidence highlighting the common incidence and source of key modifiable PACs is an important first step to raising awareness not just amongst clinicians, but to patients and families. Education should be targeted at paying equal attention and efforts to prevent common PACs, as we do to those that are infrequent (Table 1). Implementing ICU-based rehabilitation therefore requires a paradigm shift from considering the critical illness trajectory in series where rehabilitation is traditionally reserved to the “back end” of critical care, to an integrative model where rehabilitation is considered early in the critical illness trajectory as an important part of front-end care (Figure 2). An integrative model may provide us with the best opportunity to screen for and reduce morbidity prior to the onset of PACS, and in so doing, optimise functional recovery. Understanding and improving ICU survivorship is a key focus of ongoing adult and paediatric critical care research, prompting novel interventional, quality improvement and implementation science research methods (Choong et al. ongoing research; Wieczorek et al. 2016; Nydahl et al. 2018), as well as international collaboratives in identifying core patient and family-centred outcomes, and patient and family engagement in critical care research (Needham et al. 2017; Connolly and Hough 2017).
Conclusion
The success of paediatric critical care is evidenced by significant improvements in patient survival. However, these improvements are offset by the emergence of PICU-acquired morbidities both in the short term, as well as persistent long-term patient and family sequelae. Markers of success in critical care can therefore no longer be measured by survival, but improvements in longer-term survivorship post-PICU discharge. Improved understanding of populations shifts within the PICU, increasing awareness of PACs and the post-intensive care syndrome have offered us opportunities to highlight the importance of harm reduction, education and knowledge translation around survivorship following critical illness. A paradigm shift from early recognition and resuscitation, to early recognition and the introduction of ICU-based rehabilitation strategies, may offer us opportunities to reduce harm, improve the process of care, facilitate patient and family engagement team collaboration in clinical care and critical care research, and most importantly, improve functional recovery and quality of life following critical illness.
Disclosures
Karen Choong has received funding from the Academic Health Sciences Alternate Funding Plan Innovation grant to conduct the Early Rehabilitation in Critically Ill Children (PICU Liber8) Study. (Funding reference #: HAH-17-04).
Key points
- Mortality rates in children admitted to Paediatric Intensive Care Units (PICUs) have fallen significantly to an all-time low of 1- 3% in developed nations.
- Critically ill children with pre-existing chronic co-morbidities have risen significantly; these patients now constitute 53-68% of the PICU population.
- Sleep is commonly disrupted in critically ill patients, due to numerous factors such as a disruptive environment, invasive interventions, interruptions for nursing care, pain related to the underlying illness and pharmacological interventions.
- Despite its increasing incidence, PACs continue to be under-recognised amongst PICU clinicians.
- The rise in PICU-acquired complications are not only due to increasing complexity and co-morbidities amongst the critically ill paediatric population, but in large part attributable to a traditional practice paradigm of excessive sedation, prolonged immobility, knowledge gaps with respect to the risk of our current practice and subsequently, a lack of standardised strategies for the monitoring of and prevention of these sequelae.
Anand KJ, Willson DF, Berger J, et al.(2010) Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics, 125(5):e1208-1225.
Briassoulis G, Filippou O, Natsi L, Mavrikiou M, Hatzis T et al (2004) Acute and chronic paediatric intensive care patients: current trends and perspectives on resource utilization. QJM, 97(8):507-518.
Burns JP, Sellers DE, Meyer EC, Lewis-Newby M, Truog RD et al. (2014) Epidemiology of death in the PICU at five U.S. teaching hospitals. Crit Care Med,42(9):2101-2108.
Choong K, Fraser D, Al-Harbi S, et al. (2018) Functional Recovery in Critically Ill Children, the "WeeCover" Multicenter Study. Pediatr Crit Care Med, 19(2):145-154.
Choong K, Koo KK, Clark H, et al. (2013) Early Mobilization in Critically Ill Children: A Survey of Canadian Practice. Crit Care Med, 41(7):1745-1753.
Choong K, Foster G, Fraser DD, et al.(2014) Acute rehabilitation practices in critically ill children: a multicenter study. Pediatr Crit Care Med, 15(6):e270-279.
Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M et al. (2008) Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ, 338:a1655.
Creten C, Van der Zwaan S, Bankespoor R, Leroy P, Schieveld J et al. (2011) Pediatric delirium in the pediatric intensive: a systematic review and an update on key issues and research questions. Minerva Anesthesiol, 77:1099-1107.
da Silva PS, de Aguiar VE, Neto HM, de Carvalho WB et al. (2008) Unplanned extubation in a paediatric intensive care unit: impact of a quality improvement programme. Anaesthesia, 63(11):1209-1216.
Dixon-Woods M, McNicol S, Martin G. et al. (2012) Ten challenges in improving quality in healthcare: lessons from the Health Foundation's programme evaluations and relevant literature. BMJ Qual Saf, 21(10):876-884.
Early Rehabilitation in Critically ill
Children - the PICU Liber8 Study. ClinicalTrialsgov Identifier:
NCT03573479. Choong K, et al, on behalf of
the Canadian Critical Care Trials Group.
Fink EL, Beers SR, Houtrow AJ, et al. (2019)
Early Protocolized Versus Usual Care Rehabilitation for
Pediatric Neurocritical Care Patients: A
Randomized Controlled Trial. Pediatr Crit Care Med.
Flaigle MC, Ascenzi J, Kudchadkar SR. (2016)
Identifying Barriers to Delirium Screening and Prevention in
the Pediatric ICU: Evaluation of PICU Staff
Knowledge. J Pediatr Nurs, 31(1):81-84.
Garcia Guerra G, Joffe AR, Cave D, et al.
Survey of Sedation and Analgesia Practice Among Canadian
Pediatric Critical Care Physicians. Pediatr Crit Care Med. 2016;17(9):823-830.
Goodacre S, Campbell M, Carter A. What do
hospital mortality rates tell us about quality of care?
Emergency
medicine journal : EMJ. 2015;32(3):244-247.
Hartmann
M, Saeed M, Bennett TD, Typpo K, Matos R, Olsen M. Readmission and Late
Mortality after
Critical Illness in Childhood. Pediatr Crit Care Med. 2017;18(3):e112-e121.
Ista E, van Dijk M, Gamel C, Tibboel D, de
Hoog M. Withdrawal symptoms in children after long-term
administration of sedatives and/or
analgesics: a literature review. "Assessment remains troublesome".
Intensive
Care Med. 2007;33(8):1396-1406.
Kawai Y, Rohlik G, Neu L, et al. PICU
Liberation collaborative: Bundle to eliminate delirium improves ICU
culture and outcomes. Critical Care Medicine. 2018;46(1 (Suppl)):Abstract no. 1309.
Koo K, Choong K, Fan E. Prioritizing
Rehabilitation Strategies in the Care of the Critically Ill. Critical Care
Rounds.
2011;8(4):1-5.
Kudchadkar SR, Aljohani OA, Punjabi NM.
Sleep of critically ill children in the pediatric intensive care
unit: a systematic review. Sleep medicine reviews. 2014;18(2):103-110.
Kudchadkar SR, Yaster M, Punjabi NM.
Sedation, sleep promotion, and delirium screening practices in
the care of mechanically ventilated
children: a wake-up call for the pediatric critical care community*.
Crit
Care Med. 2014;42(7):1592-1600.
Kukreti V, Shamim M, Khilnani P. Intensive
care unit acquired weakness in children: Critical illness
polyneuropathy and myopathy. Indian J Crit Care Med. 2014;18(2):95-101.
LaRosa JM, Aponte-Patel L. Iatrogenic
Withdrawal Syndrome: a Review of Pathophysiology, Prevention,
and Treatment. Current Pediatrics Reports. 2019;7(1):12-19.
Long D, Williams T. PICU delirium and
sedation knowledge, atittudes and perceptions. Australian Critical
Care.
2016;29:122-123.
Merritt C, Menon K, Agus MSD, et al. Beyond
Survival: Pediatric Critical Care Interventional Trial
Outcome Measure Preferences of Families and
Healthcare Professionals. Pediatr Crit
Care Med.
2018;19(2):e105-e111.
Mody K, Kaur S, Mauer EA, et al.
Benzodiazepines and Development of Delirium in Critically Ill Children:
Estimating the Causal Effect. Crit Care Med. 2018;46(9):1486-1491.
Morris PE, Berry MJ, Files DC, et al.
Standardized Rehabilitation and Hospital Length of Stay Among
Patients With Acute Respiratory Failure: A
Randomized Clinical Trial. Jama. 2016;315(24):2694-2702.
Namachivayam P, Shann F, Shekerdemian L, et
al. Three decades of pediatric intensive care: Who was
admitted, what happened in intensive care,
and what happened afterward. Pediatr Crit
Care Med.
2010;11(5):549-555.
Needham DM, Sepulveda KA, Dinglas VD, et
al. Core Outcome Measures for Clinical Research in Acute
Respiratory Failure Survivors. An
International Modified Delphi Consensus Study. Am J Respir Crit Care
Med.
2017;196(9):1122-1130.
Nydahl P, Diers A, Gunther U, et al.
[PROtocol-based MObilizaTION on intensive care units : Design of a
cluster randomized pilot study]. Med Klin Intensivmed Notfmed. 2018;113(7):581-592.
Penk JS, Lefaiver CA, Brady CM, Steffensen
CM, Wittmayer K. Intermittent Versus Continuous and
Intermittent Medications for Pain and
Sedation After Pediatric Cardiothoracic Surgery; A Randomized
Controlled Trial. Crit Care Med. 2018;46(1):123-129.
Pinto NP, Rhinesmith EW, Kim TY, Ladner PH,
Pollack MM. Long-Term Function After Pediatric Critical
Illness: Results From the Survivor Outcomes
Study. Pediatr Crit Care Med. 2017;18(3):e122-e130.
Pollack MM, Holubkov R, Funai T, et al.
Relationship between the functional status scale and the
pediatric overall performance category and
pediatric cerebral performance category scales. JAMA
pediatrics.
2014;168(7):671-676.
Pronovost PJ, Goeschel CA, Colantuoni E, et
al. Sustaining reductions in catheter related bloodstream
infections in Michigan intensive care
units: observational study. Bmj. 2010;340:c309.
Pun BT,
Balas MC, Barnes-Daly MA, et al. Caring for Critically Ill Patients with the
ABCDEF Bundle: Results
of the ICU Liberation Collaborative in Over
15,000 Adults. Crit Care Med. 2018.
Rennick JE, Childerhose JE. Redefining
success in the PICU: new patient populations shift targets of care.
Pediatrics.
2015;135(2):e289-291.
Silver
G, Traube C, Gerber LM, et al. Pediatric delirium and associated risk factors:
a single-center
prospective observational study. Pediatr Crit Care Med. 2015;16(4):303-309.
Simone S, Edwards S, Lardieri A, et al.
Implementation of an ICU Bundle: An Interprofessional Quality
Improvement Project to Enhance Delirium
Management and Monitor Delirium Prevalence in a Single
PICU. Pediatr
Crit Care Med. 2017;18(6):531-540.
Traube
C, Silver G, Reeder RW, et al. Delirium in Critically Ill Children: An
International Point Prevalence
Study. Crit
Care Med. 2017.
Traube C, Silver G, Gerber LM, et al.
Delirium and Mortality in Critically ill Children: Epidemiology and
Outcomes of Pediatric Delirium. Crit Care Med. 2017;45(5):891-898.
Treble-Barna A, Beers SR, Houtrow AJ, et
al. PICU-Based Rehabilitation and Outcomes Assessment: A
Survey of Pediatric Critical Care
Physicians. Pediatr Crit Care Med. 2019.
Vasilevskis
EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks:
ICU-acquired delirium and weakness--crossing the quality chasm. Chest. 2010;138(5):1224-1233.
Vet NJ, Ista E, de Wildt SN, van Dijk M,
Tibboel D, de Hoog M. Optimal sedation in pediatric intensive
care patients: a systematic review. Intensive Care Med. 2013;39:1524.
Vet NJ, de Wildt SN, Verlaat CW, et al.
Short-Term Health-Related Quality of Life of Critically Ill Children
Following Daily Sedation Interruption. Pediatr Crit Care Med. 2016;17(11):e513-e520.
Visscher
M, King A, Nie AM, et al. A quality-improvement collaborative project to reduce
pressure ulcers
in PICUs. Pediatrics. 2013;131(6):e1950-1960.
Watson RS, Choong K, Colville G, et al.
Life after Critical Illness in Children-Toward an Understanding of
Pediatric Post-intensive Care Syndrome. J Pediatr. 2018.
Wieczorek B, Ascenzi J, Kim Y, et al. PICU
Up!: Impact of a Quality Improvement Program to Promote Early Mobilization in Critically Ill
Children. Ped Crit Care Med. 2016.
Yaseen M, Al-Hameed F, Osman K, et al. A
project to reduce the rate of central line associated bloodstream infection in ICU patients to a
target of zero. BMJ Qual Improv Rep. 2016;5(1).










