ICU Management & Practice, Volume 17 - Issue 3, 2017
Point-of-care ultrasound (POCUS) is now a tool used worldwide, integrating clinical assessment of the critically ill. In this review, we focus on lung, diaphragm and cardiac ultrasound in the management of the mechanically ventilated patient. Ultrasound provides useful information to assess and monitor lung aeration, to set mechanical ventilation and to early identify respiratory complications, such as pneumothorax, pneumonia and pleural effusion. Finally, we describe how to integrate ultrasound findings to manage weaning from mechanical ventilation.
In the last years, ultrasound (US) became an essential tool in the hands of the intensivist and is now recommended both for procedural guidance and diagnostic purposes. Point-of-care ultrasound (POCUS) is an immediately available and repeatable, non-irradiating bedside tool integrating the clinical examination.
While echocardiography has a longer history
in critical care and remains the most frequently used technique (Zieleskiewicz
et al. 2015), recent years were characterised by a growing interest in the
fields of lung and diaphragm US. The evolution and combination of these three
US techniques may integrate the classical approach to mechanically ventilated patients,
both for monitoring (Bouhemad et al. 2015) and diagnostic purposes (Riviello et
al. 2016), finally contributing to the titration of mechanical ventilation
(Luecke et al. 2012) and to the management of respiratory disease.
Lung aeration assessment
Lung ultrasound (LUS) semiotics is mainly composed by artefacts. A normally aerated lung is characterised by A lines: horizontal reverberation artefacts beneath the pleural line. When the ratio between air and tissue is impaired, vertical artefacts called B lines appear: their number and coalescence are proportional to lung density (Soldati et al. 2012) and loss of aeration (Via et al. 2010). Therefore a number of LUS scores based on number and type of visualised artefacts have been proposed in the last years to allow semi-quantification of lung aeration. The most frequently used score in the intensive care unit (ICU) distinguishes 6 areas per hemithorax: sternum, anterior and posterior axillary lines identify anterior, lateral and posterior regions, each divided in superior and inferior fields (Bouhemad et al. 2010; Soummer et al. 2012). In each area, a central intercostal space is examined and a score attributed according to the number and coalescence of B lines (Figure 1): A lines or ≤2 B lines correspond to normal aeration (score 0); ≥3 well-spaced B lines correspond to moderate loss of aeration (score 1); coalescent B lines correspond to severe loss of aeration (score 2); the presence of tissue-like pattern corresponds to consolidation and therefore to complete loss of aeration (score 3). The LUS score corresponds to the sum of each area’s score and ranges from 0 (all areas are well aerated) to 36 (all areas are consolidated); it showed a good correlation with extravascular lung water (Zhao et al. 2015) and computed tomography (CT) scan (Mongodi et al. 2014). It was successfully used in weaning from mechanical ventilation (Soummer et al. 2012) and guidance to fluid resuscitation (Caltabeloti et al. 2014). A re-aeration score, based on the same patterns, may also be computed and was successfully applied to assess positive end-expiratory pressure (PEEP)-induced recruitment (Bouhemad et al. 2011) and recovery after one week of antibiotic therapy in patients affected by ventilator-associated pneumonia (Bouhemad et al. 2010).
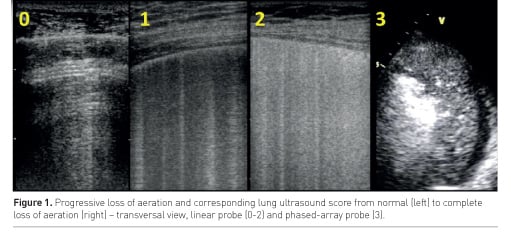
A recent study (Mongodi et al. 2017a) highlighted limitations of this score: it’s in fact suitable for homogeneous loss of aeration, when coalescence is generated by an increased number of B-lines. However, it may tend to overestimate loss of aeration when the lung pathology is non-homogeneous, such as in acute respiratory distress syndrome (ARDS), pneumonia and trauma, where focal coalescence and subpleural consolidations are frequent. To overcome this limitation, it was proposed to assign a LUS score 1 or 2 (moderate or severe loss of aeration, respectively) according to the percentage of pleura (≤ or >50%) interested by B-lines or sub-pleural consolidations (Mongodi et al. 2017a). Moreover, guidelines suggest using a longitudinal scan in order to visualise the pleura between the ribs’ shadow; however, a transversal approach aligned with the intercostal space allows visualisation of significantly wider pleura and higher number of artefacts (Mongodi et al. 2017a). Automated systems of semi-quantification are also currently under evaluation (Corradi et al. 2016).
Recruitment manoeuvre, PEEP setting and
prone position
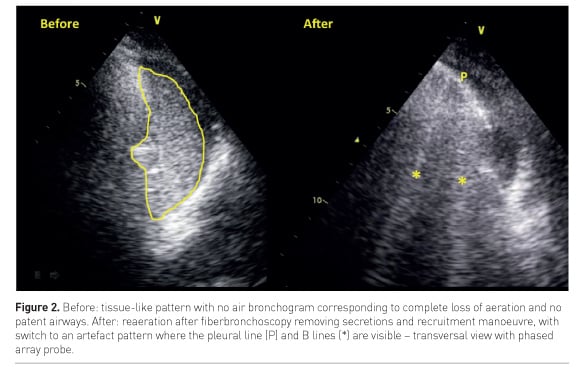
A direct monitoring of re-aeration during recruitment manoeuvre can be performed bedside by LUS (Nguyen et al, 2016): when a consolidated lung is successfully re-aerated by the manoeuvre, a real-time switch from a tissue-like pattern to an artefact pattern can be visualised, corresponding to the increase of air within the lung area (Figure 2).
A single group studied the assessment of PEEP-induced recruitment by LUS: when compared to pressure volume (PV) curve, a re-aeration score > 8 corresponded to a gain of 600 ml (Bouhemad et al. 2011). Moreover, the distribution of US artefacts and therefore of loss of aeration helps in predicting recruitment: as already assessed by CT (Constantin et al. 2010), patients with focal loss of aeration, thus with normal anterior fields, are classified as non-recruiter; high PEEP and recruitment manoeuvres are here contraindicated. On the other hand, patients with diffuse loss of aeration (i.e. affecting also anterior fields) may positively respond to recruitment and an US-monitored PEEP trial is recommended.
When patients are classified as
non-responder to PEEP, they may positively respond to prone position (Prat et
al. 2015). Patients with focal loss of aeration, compared with those with a
diffuse disease, showed in fact a greater improvement of aeration in posterior lung
areas during pronation (Haddam et al. 2016). A significant re-aeration of
posterior fields during the first cycle of pronation identified “long-term”
responders to prone position, defined by a P/F (pO2/FIO2) ratio >300 mmHg
after 7 days of 6-hour prone position twice daily (Wang et al. 2016). In this
experience, re-aeration assessed by US was associated with the decrease of dead
space and also with better outcome, a finding consistent with previous
observations (Gattinoni et al. 2003).
Heart-lung interaction
High-pressure mechanical ventilation has some drawbacks: not only does it expose to barotrauma and higher risk of complications, but it may also have a negative impact on gas exchange and haemodynamics due to lung over-distention and right ventricle (RV) impairment (Repessé et al. 2015).
Overdistention mainly affects anterior fields, impairing both alveolar ventilation and vascularisation, favouring perfusion of nonaerated regions (i.e. shunt) thus worsening respiratory acidosis and hypoxaemia. Overdistention cannot be formally measured by US; however, a reduction of physiological pleural sliding in anterior regions may be observed while increasing airway pressure (Markota et al. 2016; Pesenti et al. 2016).
You might also like: Utility of Brain Ultrasound in Neurocritical care
Moreover, an increase of airway pressure may substantially increase RV afterload. This may complicate a scenario already characterised by pulmonary vasoconstriction induced by hypoxaemia and respiratory acidosis eventually leading to a significant worsening of haemodynamics (Mongodi et al. 2017b). Incidence of acute cor pulmonale (ACP) in ARDS ranges from 14 to 50% (Repessé et al. 2015; Mekontso Dessap et al. 2016).
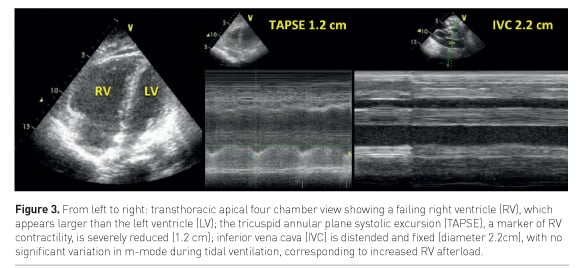
ACP may be detected by transthoracic echocardiography (TTE) as an enlarged RV (right to left ventricle end-diastolic area ratio >0.6, Figure 3) and a paradoxical septal motion (Repessé et al. 2015).
Pulmonary hypertension secondary to increased
airway pressure may lead to a rightto- left shunt through a previously silent
patent foramen ovale (PFO), significantly worsening hypoxaemia (Mekontso Dessap
et al. 2010; Mongodi et al. 2017c). The gold standard for patent PFO diagnosis
is transoesophageal echocardiography; however, TTE examination with bubble test
presents high specificity and reliably identifies significant shunts (Mojadidi et
al. 2014). Only rare cases of PFO paradoxical response to PEEP are reported in
the literature (Tavazzi et al. 2016).
Prone position may help in unloading the RV by correcting the factors inducing pulmonary vasoconstriction (i.e. hypoxaemia, hypercarbia) with no increase of airway pressure (Vieillard-Baron et al. 2007).
Therefore, a combination of lung and
heart US findings may help in setting the best PEEP level, considering both
lung aeration and haemodynamic impact.
Respiratory complications in mechanically ventilated patients
Pneumothorax
Pneumothorax can be a consequence of barotrauma, mainly when lung compliance is reduced. LUS is accurate in the diagnosis of pneumothorax (Lichtenstein et al. 2000) and is superior to supine anterior chest x-ray (Blaivas et al. 2005). If a pneumothorax is suspected, LUS should be performed following a simple algorithm (Mongodi et al. 2016a). The presence of real images (consolidation, effusion), of any pleural movement (sliding, lung pulse) or artefacts deriving from the visceral pleura (B-lines) rules out pneumothorax with 100% negative predictive value. If a static A pattern is visualised, a lung point must be searched moving the probe laterally and inferiorly: the lung point corresponds to the site where the collapsed lung goes back in touch with the parietal pleura and rules in pneumothorax with 100% positive predictive value. If no lung point is identified, the positive predictive value of a static A pattern alone ranges from 55 to 98%, depending on the clinical context; for example, if the lung is completely collapsed, no lung point can be visualised.
Although the exact size of the pneumothorax cannot be measured by US, an estimation of the percentage of collapsed lung may be suggested by the location of the lung point on the chest wall: if below the mid-axillary line in supine patients, it corresponds to lung collapse >15% with sensitivity 83.3% and specificity 82.4% when compared with CT scan (Volpicelli et al. 2014).
Depending on the expected percentage of lung collapse and on the patient’s clinical stability, LUS may guide the intensivist in the decision to drain the air collection, while allowing visualisation of parietal vessels to be avoided (Mongodi et al. 2016a).
Pleural effusion
Effusions are visualised by US as anechoic areas between the parietal and visceral pleura. US also provides morphologic assessment, suggesting the possible aetiology of fluid collection, and allows estimation of the amount of fluid that can be drained (Balik et al. 2006). Finally, US is a reliable guide to thoracentesis (Lichtenstein et al. 1999).
Consolidations
LUS accurately identifies lung consolidation and, being dynamic, is a useful tool to distinguish consolidation aetiologies by looking at air bronchograms (Berlet et al. 2015).
Air bronchogram is visualised within a consolidation as white hyperechoic spots due to air trapped inside the bronchi. When it moves during tidal ventilation—the so-called dynamic air bronchogram—the airway is patent but alveoli are not: therefore ventilatorassociated pneumonia can be suspected.
While consolidation alone is poorly specific (Zagli et al. 2014), two ultrasonographic signs of VAP have been identified: subpleural consolidations and dynamic linear/arborescent air bronchogram. These signs were combined with a clinical parameter (purulent secretions) into a simple score (Ventilator-associated Pneumonia Lung Ultrasound Score), which performed better than the classical Clinical Pulmonary Infection Score, even when combined to direct exam of tracheal aspirate (Mongodi et al. 2016b). Moreover, LUS allows monitoring of antibiotic effects: the computation of the LUS re-aeration score after one week of antibiotic treatment distinguishes responders from non-responders, eventually redirecting the therapeutic approach (Bouhemad et al. 2010).
On the other hand, when the bronchogram is absent or static, i.e. not moving during tidal ventilation, the consolidation presents airway obstruction. In fact, air cannot enter the bronchi during ventilation and the mechanism leading to loss of aeration might be reabsorption atelectasis. Fiberbronchoscopy is here indicated to clear the bronchi and restore normal airflow and lung aeration (Figure 2).
Finally, the impact on gas exchanges of a given consolidation depends on perfusion of the non-aerated tissue, i.e. intrapulmonary shunt; to grossly assess shunt, blood flow can be visualised by applying colour Doppler on the tissue-like pattern (Mongodi et al. 2016c).
Diaphragmatic dysfunction
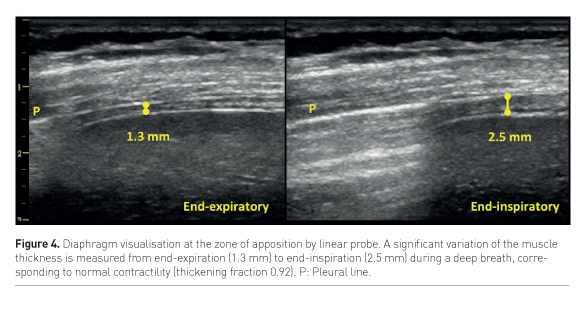
US can assess both diaphragm morphology and function by measuring its thickness, excursion and thickening fraction. The muscle thickness is a static measurement obtained by examining the diaphragm with a linear probe at the zone of apposition to the thoracic wall, where the diaphragm is visualised as a double binary structure parallel to the probe. It’s a very reproducible measure (Goligher et al. 2015a), more easily obtained on the right side, and provides significant information about the muscle status. It has been shown that diaphragm thickness progressively declines during mechanical ventilation, being a marker of the increasing atrophy of the muscle, especially in cases of controlled mechanical ventilation or high-level assisted mechanical ventilation (Schepens et al. 2015; Hudson et al. 2012). Diaphragm excursion consists in muscle dome caudal displacement during inspiration (Kim et al. 2011), and can be quantified by m-mode technique with a phased-array probe. This measurement presents two main limitations: first, m-mode may not be aligned with the course of the diaphragm, thus leading to underestimation of the excursion. This can be avoided by the use of the anatomical m-mode (Pasero et al. 2015). Second, diaphragm excursion in ventilated patients is affected by the pressure support delivered by the ventilator; active and passive displacement cannot be distinguished. Instead, active contractility can be detected as an increase in muscle thickness during respiratory efforts (Figure 4), and the diaphragm thickening ratio, i.e. (thickness at end-inspiration – thickness at end-expiration) / thickness at end-expiration, can be used as a measure of muscle activity. Ideally, to avoid both disuse atrophy and stress injury of the diaphragm, during assisted ventilation the level of support could be titrated according to diaphragm activity assessed by US. (Goligher et al. 2015b).
Weaning from mechanical ventilation
The weaning process from mechanical ventilation covers up to 40-50% of total mechanical ventilation time (McConville and Kress 2012). Weaning failure ranges from 25 to 61% (Esteban et al. 1999), depending on the clinical context; multiple physiopathological mechanisms may be involved, therefore weaning process remains a significant challenge for the intensivist. Three of the main mechanisms leading to weaning failure can be assessed by US: cardiac dysfunction, lung derecruitment and diaphragm dysfunction.
While no impact of systolic parameters has been found, diastolic assessment by transmitralic pattern and tissue Doppler of mitral annulus can identify the failing patient with moderate sensitivity and high specificity (Moschietto et al. 2012). The assessment of lung aeration score at the end of a spontaneous breathing trial predicts extubation failure with 0.86 area under curve (AUC) (Soummer et al. 2012). Finally, respiratory muscles dysfunction as assessed by diaphragm US during a spontaneous breathing trial or low-pressure support ventilation is associated with weaning failure. (Kim et al. 2012; Spadaro et al. 2016; Blumhof et al. 2016).
A combined US approach assessing lung, heart and diaphragm has been suggested, not only for early identification of the failing patient but also for a better understanding of the underlying mechanism of failure, thus guiding therapeutic management to improve weaning success rate (Mongodi et al. 2013; Mayo et al. 2016).
Conclusions
Lung, diaphragm and cardiac US provide significant information to improve the management of the critical patient under mechanical ventilation, from the initial assessment, through the ventilation setting and its complication diagnosis, until the weaning process.
Conflict of interest
Francesco Mojoli received fees for lectures from GE healthcare and for lectures and consultancy from Hamilton Medical. Silvia Mongodi declares that she has no conflict of interest.
Abbreviations
ARDS acute respiratory distress syndrome
ICU intensive care unit
LUS lung ultrasound
PFO patent foramen ovale
PEEP positive end-expiratory pressure
RV right ventricle
US ultrasound
VAP ventilator-associated pneumonia
RV right ventricle
TTE transthoracic echocardiography
Tweets
References:
Balik M, Plasil P, Waldauf P et al. (2006) Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med, 32(2): 318.
Berlet T, Etter R, Fehr T et al. (2015) Sonographic patterns of lung consolidation in mechanically ventilated patients with and without ventilator-associated pneumonia: a prospective cohort study. J Crit Care, 30(2): 327-33.
Blaivas M, Lyon M, Duggal S (2005) A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med, 9: 844-9.
Blumhof S, Wheeler D, Thomas K et al. (2016) Change in diaphragmatic thickness during the respiratory cycle predicts extubation success at various levels of pressure support ventilation. Lung, 194(4): 519-25.
Bouhemad B, Liu Z, Arbelot C et al. (2010) Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med, 38(1): 84-92.
Bouhemad B, Brisson H, Le-Guen M et al. (2011) Bedside ultrasound assessment of positive endexpiratory pressure-induced lung recruitment. Am J Respir Crit Care Med, 183(3): 341-7.
Bouhemad B, Mongodi S, Via, G et al. (2015) Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology, 122: 437-47.
Caltabeloti F, Monsel A, Arbelot C et al. (2014) Early fluid loading in acute respiratory distress syndrome with septic shock deteriorates lung aeration without impairing arterial oxygenation: a lung ultrasound observational study. Crit Care, 18(3): R91.
Constantin JM, Futier E, Cherprenet AL et al. (2010) A recruitment maneuver increases oxygenation after intubation of hypoxemic intensive care unit patients: a randomized controlled study. Crit Care, 14(2): R76.
Corradi F, Brusasco C, Vezzani A et al. (2016) Computer-aided quantitative ultrasonography for detection of pulmonary edema in mechanically ventilated cardiac surgery patients. Chest, 150(3): 640-51.
Esteban A, Alía I, Tobin MJ et al. (1999) Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med, 159(2): 512-8.
Gattinoni L, Vagginelli F, Carlesso E et al. (2003) Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med, 31(12): 2727-33.
Goligher EC, Laghi F, Detsky ME et al. (2015a) Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med, 41(4): 642-9.
Goligher EC, Fan E, Herridge MS et al. (2015b) Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. Am J Respir Crit Care Med, 192(9): 1080-8.
Haddam M, Zieleskiewicz L, Perbet S et al. (2016) Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med, 42(10): 1546-56.
Hudson MB, Smuder AJ, Nelson WB et al. (2012) Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med, 40(4): 1254-60.
Kim WY, Suh HJ, Hong SB et al. (2011) Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med, 39(12): 2627-30.
Lichtenstein D, Hulot JS, Rabiller A et al. (1999) Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med, 25(9): 955-8.
Lichtenstein D, Mezière G, Biderman P et al. (2000) The "lung point": an ultrasound sign specific to pneumothorax. Intensive Care Med, 10: 1434-40.
Luecke T, Corradi F, Pelosi P (2012) Lung imaging for titration of mechanical ventilation. Curr Opin Anaesthesiol, 25(2): 131–40.
Markota A, Golub J, Stožer A et al. (2016) Absence of lung sliding is not a reliable sign of pneumothorax in patients with high positive end-expiratory pressure. Am J Emerg Med, 34(10): 2034-6.
Mayo P, Volpicelli G, Lerolle N et al. (2016) Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med, 42(7): 1107-17.
McConville JF, Kress JP (2012) Weaning patients from the ventilator. N Engl J Med, 367(23): 2233-9.
Mekontso Dessap A, Boissier F, Leon R et al. (2010) Prevalence and prognosis of shunting across patent foramen ovale during acute respiratory distress syndrome. Crit Care Med, 38(9): 1786-92.
Mekontso Dessap A, Boissier F, Charron C et al. (2016) Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med, 42(5): 862-70.
Mojadidi MK, Winoker JS, Roberts SC et al. (2014) Accuracy of conventional transthoracic echocardiography for the diagnosis of intracardiac right-to-left shunt: a meta-analysis of prospective studies. Echocardiography, 31: 1036–48.
Mongodi S, Via G, Bouhemad B et al. (2013) Usefulness of combined bedside lung ultrasound and echocardiography to assess weaning failure from mechanical ventilation: a suggestive case. Crit Care Med, 41(8): e182-5.
Mongodi S, Algieri I, Mojoli F et al. (2014) CT-scan and ultrasound comparative assessment of lung aeration in ARDS. Intensive Care Med, 40(Suppl): S127.
Mongodi S, Luperto M, Orlando A et al. (2016a) A 67-year-old man with severe posttraumatic ARDS in extracorporeal membrane oxygenation presents sudden desaturation. Chest, 150(6): e155-e157.
Mongodi S, Via G, Girard M et al. (2016b) Lung ultrasound for early diagnosis of ventilator-associated pneumonia. Chest, 149(4): 969-80.
Mongodi S, Bouhemad B, Iotti GA et al. (2016c) An ultrasonographic sign of intrapulmonary shunt. Intensive Care Med, 42(5): 912-3.
Mongodi S, Bouhemad B, Orlando A et al. (2017a) Modified lung ultrasound score for assessing and monitoring pulmonary aeration. Ultraschall Med, 14 Mar. doi: 10.1055/s-0042-120260. [Epub ahead of print]
Mongodi S, Orlando A, Tavazzi G et al. (2017b) Veno-venous extracorporeal membrane oxygenation for acute respiratory distress syndrome in a patient with acute right heart failure. J Cardiothorac Vasc Anesth, 31(4): 1374-7.
Mongodi S, Via G, Riccardi M et al. (2017c) Patent foramen ovale diagnosis: The importance of provocative maneuvers. J Clin Ultrasound, 45(1): 58-61.
Moschietto S, Doyen D, Grech L et al. (2012) Transthoracic echocardiography with Doppler tissue imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care, 16(3): R81.
Nguyen M, Benkhadra S, Douguet C et al. (2016) Real-time visualization of left lung consolidation relief using lung ultrasound. Am J Respir Crit Care Med, 193(11): e59-60.
Pasero D, Koeltz A, Placido R et al. (2015) Improving ultrasonic measurement of diaphragmatic excursion after cardiac surgery using the anatomical M-mode: a randomized crossover study. Intensive Care Med, 41(4): 650-6.
Pesenti A, Musch G, Lichtenstein D et al. (2016) Imaging in acute respiratory distress syndrome. Intensive Care Med, 42(5): 686-98.
Prat G, Guinard S, Bizien N et al. (2015) Can lung ultrasonography predict prone positioning response in acute respiratory distress syndrome patients? J Cri Care, 32: 36–41.
Repessé X, Charron C, Vieillard-Baron A (2015) Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest, 147(1): 259-65.
Riviello E, Kiviri W, Twagirumugabe T et al. (2016) Hospital incidence and outcomes of the acute respiratory distress syndrome using the Kigali modification of the Berlin definition. Am J Respir Crit Care Med, 193(1): 1546-56.
Schepens T, Verbrugghe W, Dams K et al. (2015) The course of diaphragm atrophy in ventilated patients assessed with ultrasound: a longitudinal cohort study. Crit Care, 7(19): 422.
Soldati G, Inchingolo R, Smargiassi A et al. (2012) Ex vivo lung sonography: morphologic-ultrasound relationship’, Ultrasound Med Biol, 38(7): 1169-79.
Soummer A, Perbet S, Brisson H et al. (2012) Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med, 40(7): 2064-72.
Spadaro S, Grasso S, Mauri T et al. (2016) Can diaphragmatic ultrasonography performed during the T-tube trial predict weaning failure? The role of diaphragmatic rapid shallow breathing index. Crit Care, 20(1): 305.
Tavazzi G, Pozzi M, Via G et al. (2016) Weaning failure for disproportionate hypoxemia caused by paradoxical response to positive end-expiratory pressure in a patient with patent foramen ovale. Am J Respir Crit Care Med, 193(1): e1–2.
Via G, Lichtenstein D, Mojoli F et al. (2010) Whole lung lavage: a unique model for ultrasound assessment of lung aeration changes. Intensive Care Med, 36(6): 999-1007.
Vieillard-Baron A, Charron C, Caille V et al. (2007) Prone positioning unloads the right ventricle in severe ARDS. Chest, 132(5): 1440-6.
Volpicelli G (2014) A title is worth a thousand words: it is possible to semi-quantify pneumothorax by lung ultrasound. Intensive Care Med, 40 (10): 1616-7.
Wang XT, Ding X, Zhang HM et al. Chinese Critical Ultrasound Study Group (CCUSG) (2016) Lung ultrasound can be used to predict the potential of prone positioning and assess prognosis in patients with acute respiratory distress syndrome. Crit Care 20(1): 385.
Zagli G, Cozzolino M, Terreni A et al. (2014) Diagnosis of ventilator-associated pneumonia: a pilot, exploratory analysis of a new score based on procalcitonin and chest echography. Chest, 146(6): 1578-85.
Zhao Z, Jiang L, Xiuming X et al. (2015) Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm Med, 15: 98.
Zieleskiewicz L, Muller L, Lakhal K et al. (2015) Point-of-care ultrasound in intensive care units: assessment of 1073 procedures in a multicentric, prospective, observational study. Intensive Care Med, 41: 1638-47.