ICU Management & Practice, Volume 16 - Issue 1, 2016
The aim of the present review is to summarise the current evidence for the use of biomarkers in facilitating therapeutic decision-making by guiding and tailoring the prescription and the duration of antibiotic therapy. The main benefits of this strategy are a potential reduction of antibiotics overuse in critically ill patients.
Overuse of antibiotics and its consequences represent a big challenge for healthcare services worldwide. One reason is the change in profile of inpatients observed during the last decades: they are increasingly older, have more comorbidities and are frequently immunosuppressed (Zuckerman et al. 2007). As a consequence, multidrug-resistant organisms are becoming very common as the aetiology of healthcare associated infections (Martin-Loeches et al. 2014), and frequently these pathogens are isolated in patients with infection acquired in the outpatient setting. Therefore, we have in combination the difficulty of prescribing antibiotics to more vulnerable hosts with more difficult to treat infections. Clinical signs and symptoms are in general of limited value. As a result the prescription of antibiotics, often a combination of broad-spectrum agents, is done in an early and empirical fashion (Klein Klouwenberg et al. 2015). However, administration of unnecessary antibiotics will develop bacterial resistance against these drugs.
Currently, the most important aspect to improve outcome is early recognition of infection and prompt initiation of appropriate empiric antibiotic treatment. Initiation too late or inappropriate antibiotic therapy is associated with adverse outcomes (Ferrer et al. 2014). In addition there is an unmet need for data regarding when to stop antibiotics in two particular clinical scenarios:
- It is not clear which is the best time point to reassess a patient in order to define if an infectious process is present.
- To define when the patient is not responding to the antibiotic being administered without reaching clinical stability or, oppositely, to define when infection is clinically cured and antibiotics could be stopped.
A biomarker or biological marker generally refers to a measurable indicator of some biological state or condition (Martin-Loeches et al. 2015). In the context of infection, the ideal biological marker would discriminate when a patient needs to discontinue or de-escalate an antibiotic course.
Evidence Against
It is now clear that at least for patients with severe infections the timing of antibiotic onset is markedly associated with the outcome (Kumar et al. 2006; Ferrer et al. 2014). However, data concerning the ideal duration of antibiotic therapy is scarce, even though growing evidence from recent studies suggests that short courses of treatment are effective and safe. Interestingly, most of these studies did not use biomarkerguided protocols to reduce antibiotic use.
Two landmark studies specifically evaluated the impact of fixed durations of antibiotic therapy to treat severe infections in intensive care unit (ICU) patients (Chastre et al. 2003; Micek et al. 2004). The PneumA trial (Chastre et al. 2003) was a prospective randomised controlled trial (RCT) in 51 French ICUs, designed to assess whether 8 days was as effective as 15 days of antibiotic therapy in microbiologically documented late-onset ventilator-associated pneumonia (VAP) (n=402). No difference in the all-cause mortality rate was observed between the two groups (18.8% vs. 17.2%). In the subgroup of patients with VAP caused by non-fermentative Gram-negative bacilli, patients treated for 8 days had higher rates of recurrence of infection as compared to the longer treatment group (40.6% vs. 25.4%, p<0.05), but with no excess of mortality (23.4% vs. 30.2%, p=NS). In addition, the rate of emergence of multidrug-resistant bacteria was significantly lower in the 8-day therapy group (41.2% vs. 62.3%, p=0.04).
The second study (Micek et al. 2004) was a single-centre prospective RCT designed to evaluate the effectiveness and safety of an active discontinuation policy for the duration of antibiotic therapy for VAP (n=290). The active discontinuation policy consisted of a) initial administration of adequate antibiotic treatment and b) antibiotics should be discontinued if b.1) the infiltrates were considered to be non infectious aetiology or b.2) the signs and symptoms suggested that the active infection had resolved. The authors showed that the active discontinuation policy could safely decrease the duration of antibiotic therapy to 6 days in comparison to the standard of care, which was 8 days (p=0.001). Both groups presented comparable clinical outcomes, namely: hospital mortality, ICU and hospital length of stay (LOS) (p=0.357, p=0.798, p=0.865, respectively). In addition, the rate of VAP recurrence was similar in both groups (p=0.667).
Similar data have been published in other relevant clinical situations, such as community-acquired pneumonia and non-complicated pyelonephritis (Li et al. 2007; Sandberg et al. 2012). Additional benefits demonstrated by these studies were lower emergence of bacterial resistance, better adherence to treatment, decreased toxicity and reduced costs.
A randomised controlled trial (RCT) on non-critically ill patients with complicated intra-abdominal infections demonstrated that, in patients with adequately controlled infections, a fixed short course of antibiotics (median duration of 4 days) produced similar outcomes, namely rate of septic complications, as compared to 10 days of therapy (Sawyer et al. 2015). Taken together, these findings all suggest that, even in ICU patients with severe infections, namely VAP and intra-abdominal infection, the duration of antibiotic therapy can be safely shortened to 6-8 days, regardless of the use of biomarkers.
Evidence in Favour
As far as we are aware there are 11 RCTs of procalcitonin (PCT)-guided antibiotic therapy in critically ill patients for initiation and cessation of antibiotic therapy or both (Svoboda et al. 2007; Nobre et al. 2008; Schroeder et al. 2009; Stolz et al. 2009; Hochreiter et al. 2009; Bouadma et al. 2010; Jensen et al. 2011; Layios et al. 2012; Deliberato et al. 2013; Annane et al. 2013; Shehabi et al. 2014). The relative quality of the majority of these RCTs has been discussed in detail elsewhere ( Póvoa and Salluh 2012).
Despite its limitations, these trials make PCT the most studied biomarker to guide antibiotic therapy in critically ill patients (Table 1). Concerning the decision to start antibiotics (Svoboda et al. 2007; Bouadma et al. 2010; Jensen et al. 2011; Layios et al. 2012; Annane et al. 2013), not a single trial was able to demonstrate that PCT-guided initiation of antibiotic therapy constitutes a helpful strategy in comparison to current clinical judgment for decreasing antibiotic consumption in critically ill patients. This could be related to the fact that clinicians already have a low threshold to start antibiotics and that, at least in critically ill patients, biomarkers are unlikely to favourably change that threshold.
On the contrary, with the exception of two RCTs (Jensen et al. 2011; Annane et al. 2013), all the other PCT-guided antibiotic stewardship trials showed that using very similar PCT-algorithms was associated with a shorter duration of antibiotic therapy. Of note, this reduction was achieved with no apparent harm; i.e., there were comparable ICU LOS, relapse/recurrence rates of infection as well as mortality rate between patients of the PCT-guided group and controls (Table 1).
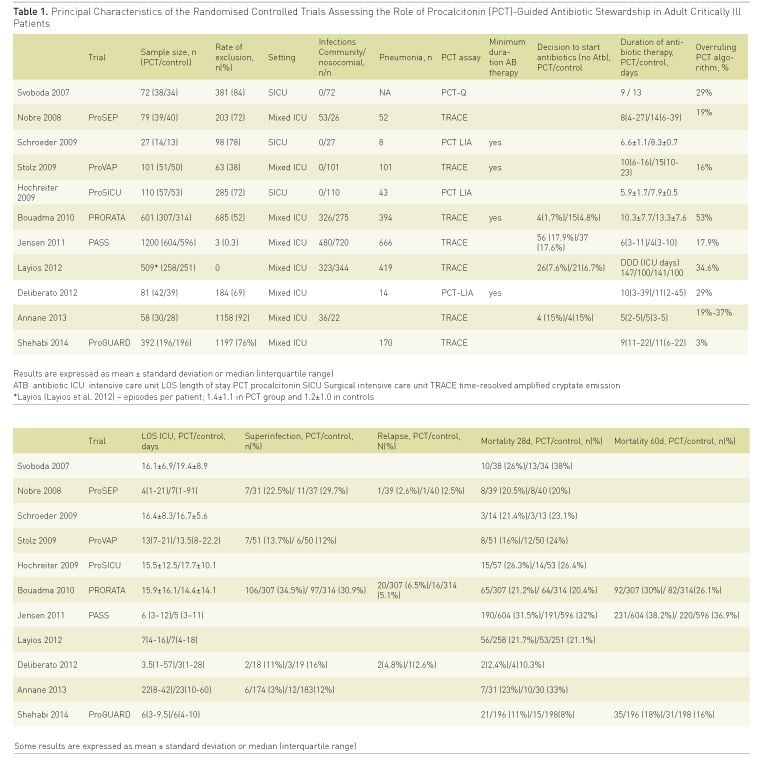
In a recent meta-analysis (Schuetz et al. 2012) it was clear that for ventilator-associated pneumonia (VAP), use of PCT-guided algorithms allowed a reduction of 3 days (from 14 to 11 days) in the mean duration of antibiotic therapy. However, in some trials the shortening of the antibiotic therapy courses was achieved due to excessively long and fixed antibiotic treatments in the control groups (Póvoa and Salluh 2012). This strategy, i.e., fixed and long courses of antibiotics in the control groups, may have led to an ‘artificial’ reduction of antibiotic treatment in the PCT-based arms. In addition, these trial designs also did not take into account data available for more than a decade from trials showing that a 6 to 8-day course of antibiotics is effective and safe to treat severe infections (Chastre et al. 2003;Micek et al. 2004).
How Can we Properly Use Biomarkers?
With the previously mentioned limitations in mind, we should ask: how can we properly use biomarkers such as PCT and C-reactive protein (CRP) to guide antimicrobial therapy (initiation and duration) in severe sepsis? (Vincent and Teixeira 2014). First, we believe that future clinical RCTs should use less strict entry criteria that would better reflect our real-life ICU patients with sepsis. Second, a great deal of effort must be put into conducting multicentre studies, involving a large number of patients, ideally in different regions of the world. Lastly, biomarker-guided strategies must be tested against a comparator that actually reflects the “best care” (i.e., implementation of the best available evidence), which increasingly balances toward shorter courses of antibiotic therapy instead of the highly variable, and usually longer than necessary, “standard care”. In our opinion, it means comparing PCT-algorithms with control treatment in which the maximum duration of antibiotic therapy is set up in 7-8 days (Chastre et al. 2003; Micek et al. 2004). Until these studies are performed and their results become available, clinicians should perhaps use a “double-trigger” strategy as proposed by Oliveira et al. (2013). In this RCT, in which a PCT-guided protocol was compared to a CRP-guided strategy, antibiotics were stopped according to clinical response to therapy associated with either a pre-established reduction in the circulating levels of these biomarkers or the completion of 7 full days of treatment, whichever comes first. In this single-centre study that evaluated patients with severe sepsis and septic shock, PCT and CRP were similarly effective in ensuring an early interruption of antibiotics [7(6-8.5) vs. 6(5-7) days, respectively].
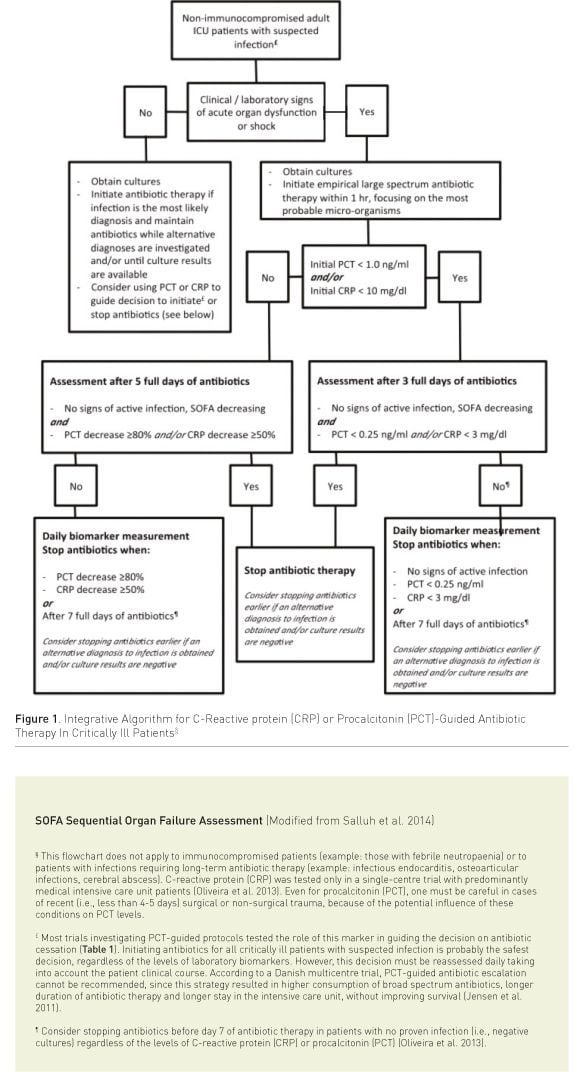
Proposal of an Integrative Algorithm
Considering the critical revision of present data, we propose an integrative algorithm, incorporating the available evidence on using PCT or CRP-guided strategies, in addition to clinical and laboratory information, to reduce antibiotic therapy in critically ill patients. Our proposal is presented in Figure 1 (Salluh et al. 2014). It must be stressed that biomarkers represent only one of the available tools to promote antibiotic stewardship and should never be used as a single tool. Additional measures to reduce inappropriate antibiotic exposure should always be considered.
Conclusion
Different strategies have been designed to implement and operationalise antibiotic stewardship in critically ill patients. Antibiotics are very powerful drugs, that when adequately and used in a timely manner make a huge difference in the prognosis of severe sepsis and septic shock patients. Antibiotics only treat infections, not sepsis-like syndromes. But it is also important to recognise that antibiotics are not innocent players; their use, in particular if prolonged and inadequate, is associated with increased duration of mechanical ventilation, LOS, mortality, recurrence of infection, toxicity, emergence of bacterial resistance and costs.
To approach this complex problem we need new approaches and helpful tools. A strategy like the one we propose, although not validated, puts together valuable and reliable information from different RCTs. This algorithm takes into consideration a “double-trigger” strategy (time/ clinic course and biomarker level) and could be implemented as a helpful tool in the daily clinical decision making process at the bedside.
Conflict of Interest
Pedro Póvoa has unrestricted research grants from
ThermoFisher Scientific and Virogates. The other authors state that they have
no competing interest with the subject.
Abbreviations
CRP C-reactive protein
LOS length of stay
PCT procalcitonin
RCT randomised controlled trial
References:
Annane D, Maxime V, Faller JP et al. (2013) Procalcitonin levels to guide antibiotic therapy in adults with non-microbiologically proven apparent severe sepsis: a randomised controlled trial. BMJ Open, 14(3): 2. PubMed↗
Bouadma L, Luyt CE, Tubach F et al. (2010) Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet, 375(9713): 463-74. PubMed↗
Chastre J, Wolff M, Fagon JY et al. (2003) Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA, 290(19): 2588-98. PubMed↗
Deliberato RO, Marra AR, Sanches PR et al. (2013) Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis, 76(3): 266-71. PubMed↗
Ferrer R, Martín-Loeches I, Phillips G et al. (2014) Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med, 42(8): 1749-55. PubMed↗
Hochreiter M, Köhler T, Schweiger AM et al. (2009) Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care, 13(3): R83. PubMed↗
Jensen JU, Hein L, Lundgren B et al. (2011) Procalcitonin-guided interventions against infections to increase early appropriate antibiotics and improve survival in the intensive care unit: a randomized trial. Crit Care Med, 39(9): 2048-58.
Klein Klouwenberg PM, Cremer OL, van Vught LA et al. (2015) Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care, 19: 319. PubMed↗
Kumar A, Roberts D, Wood KE et al. (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med, 34(6): 1589-96. PubMed↗
Layios N, Lambermont B, Canivet JL et al. (2012) Procalcitonin usefulness for the initiation of antibiotic treatment in intensive care unit patients. Crit Care Med, 40 (8): 2304-9. PubMed↗
Li J Z, Winston LG, Moore DH et al. (2007) Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med, 120(9): 783-90.
Martín-Loeches I, Bos LD, Povoa P et al. (2015) Tumor necrosis factor receptor 1 (TNFRI) for ventilator-associated pneumonia diagnosis by cytokine multiplex analysis. Intensive Care Med Exp, 3(1): 26. PubMed↗
Martín-Loeches I, Diaz E, Vallés J (2014) Risks for multidrug-resistant pathogens in the ICU. Curr Opin Crit Care, 20(5): 516-24. PubMed↗
Micek ST, Ward S, Fraser VJ et al. (2004) A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator-associated pneumonia. Chest, 125(5): 1791-9. PubMed↗
Nobre V, Harbarth S, Graf JD et al. (2008) Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med, 177(5): 498-505. PubMed↗
Oliveira CF, Botoni FA, Oliveira CR et al. (2013) Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit Care Med, 41(10): 2336-43.
Povoa P, Salluh JI (2012) Biomarker-guided antibiotic therapy in adult critically ill patients: a critical review. Ann Intensive Care, 2(1): 32.
Salluh JI, Nobre V, Povoa P (2014) Using procalcitonin to guide antimicrobial duration in sepsis: asking the same questions will not bring different answers. Crit Care, 18(3): 142. PubMed↗
Sandberg T, Skoog G, Hermansson AB et al. (2012) Ciprofloxacin for 7 days versus 14 days in women with acute pyelonephritis: a randomised, open-label and double-blind, placebo-controlled, non-inferiority trial. Lancet, 380 (9840): 484-90. PubMed↗
Sawyer RG, Claridge JA, Nathens AB et al. (2015) Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med, 372(21): 1996-2005. PubMed↗
Schroeder S, Hochreiter M, Koehler T et al. (2009) Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: results of a prospective randomized study. Langenbecks Arch Surg, 394(2): 221-6.PubMed↗
Schuetz P, Müller B, Christ-Crain M et al. (2012) Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev, 9: CD007498. PubMed↗
Shehabi Y, Sterba M, Garrett PM et al. (2014) Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. Am J Respir Crit Care Med, 190(10): 1102-10. PubMed↗
Stolz D, Smyrnios N, Eggimann P et al. (2009) Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J, 34(6): 1364-75. PubMed↗
Svoboda P, Kantorová I, Scheer P et al. (2007) Can procalcitonin help us in timing of re-intervention in septic patients after multiple trauma or major surgery? Hepatogastroenterology, 54(74): 359-63. PubMed↗
Vincent JL, Teixeira L (2014) Sepsis biomarkers. Value and limitations. Am J Respir Crit Care Med, 190(10): 1081-2.
Zuckerman IH, Perencevich EN, Harris AD (2007) Concurrent acute illness and comorbid conditions poorly predict antibiotic use in upper respiratory tract infections: a cross-sectional analysis. BMC Infect Dis, 7: 47.