ICU Management & Practice, Volume 23 - Issue 2, 2023
Heparin-induced thrombocytopaenia (HIT) is an immune complication of heparin therapy. This review discusses the pathophysiology, incidence, clinical manifestations, diagnostic approach, and management of patients with HIT.
Pathophysiology
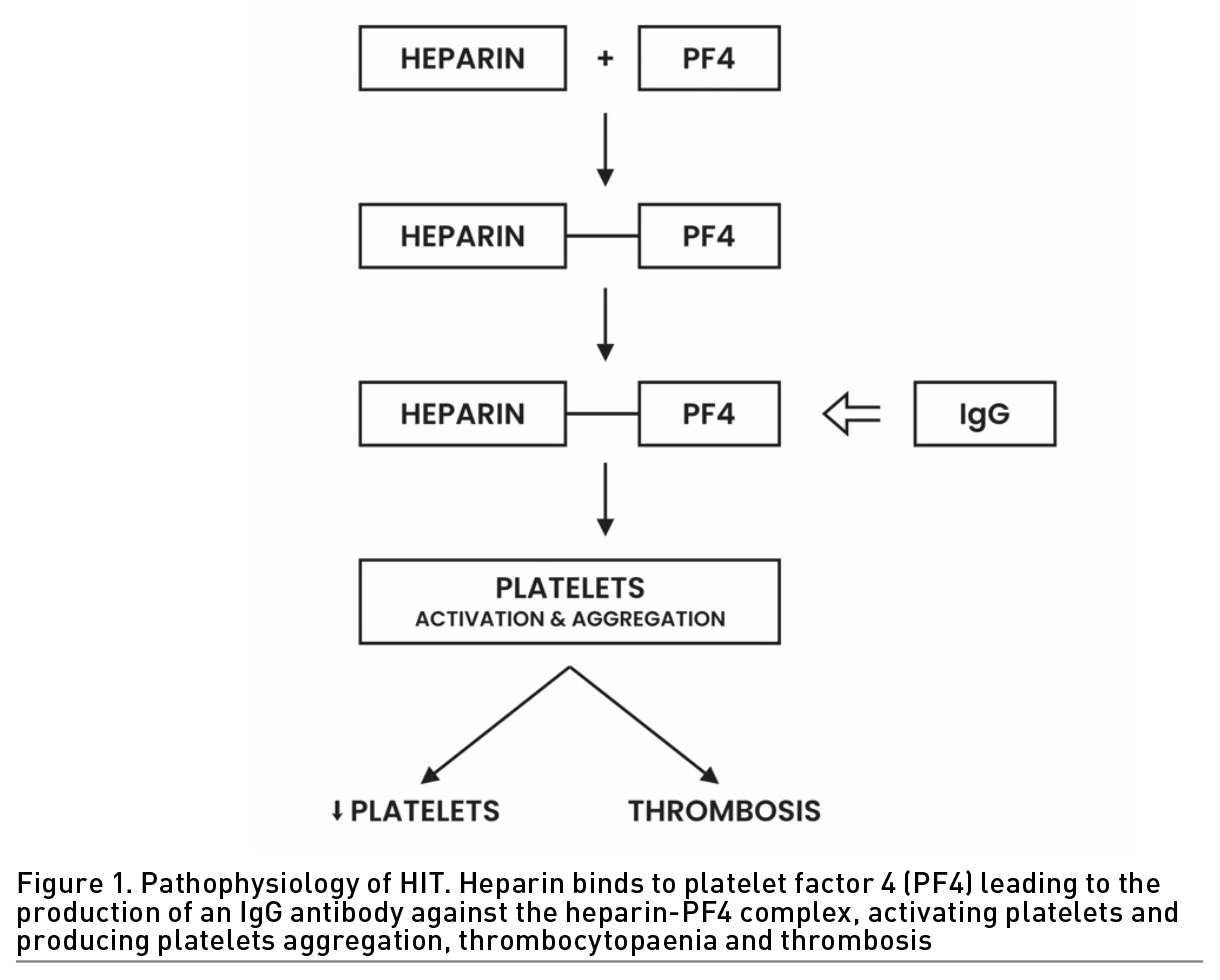
HIT is an immune complication of heparin therapy caused by IgG antibodies to complexes of platelet factor 4 (PF4) and heparin. These antibodies bind to the Fc receptor of the platelet surface, leading to platelet activation. Activation of platelets results in platelet aggregation and consumption, which results in thrombocytopaenia and thrombosis (Figure 1).
Incidence
Approximately 0.2% of patients exposed to heparin develop HIT. The incidence is higher with unfractionated heparin (UFH) compared with low molecular weight heparin (LMWH), and HIT is also more common among surgical patients than medical patients (Jevtic 2012). HIT is very uncommon in patients who receive prophylactic doses and rare in pregnant women. Patients undergoing cardiac surgery and vascular surgery and patients on haemodialysis have high rates of HIT compared to the general medical hospital population.
Clinical Manifestations
HIT should be suspected in a patient who develops thrombocytopaenia that is usually associated with thrombosis 5 to 10 days after heparin administration. In these patients, the platelet counts are decreased more than 50% from baseline, but severe thrombocytopaenia (< 20,000 x 109/L) is infrequent. Despite the thrombocytopaenia, bleeding is not classically associated with HIT. Moreover, as many as 70% of patients with HIT develop thrombosis of any vascular bed. Thrombosis may affect the venous and arterial circulation, and conditions, such as deep vein thrombosis, pulmonary embolism, cerebral dural sinus thrombosis, adrenal haemorrhagic infarction, arterial thrombosis of the upper limb, lower limb, mesenteric, renal and spinal arteries, acute thrombotic stroke, and myocardial infarction, can occur. Venous thrombosis occurs more often than arterial thrombosis, with lower limb deep venous thrombosis and pulmonary embolism being the most common. Uncommon presentations of HIT are necrotic skin lesions, either at the injection site or distant sites, and acute systemic anaphylactoid reactions.
Diagnosis
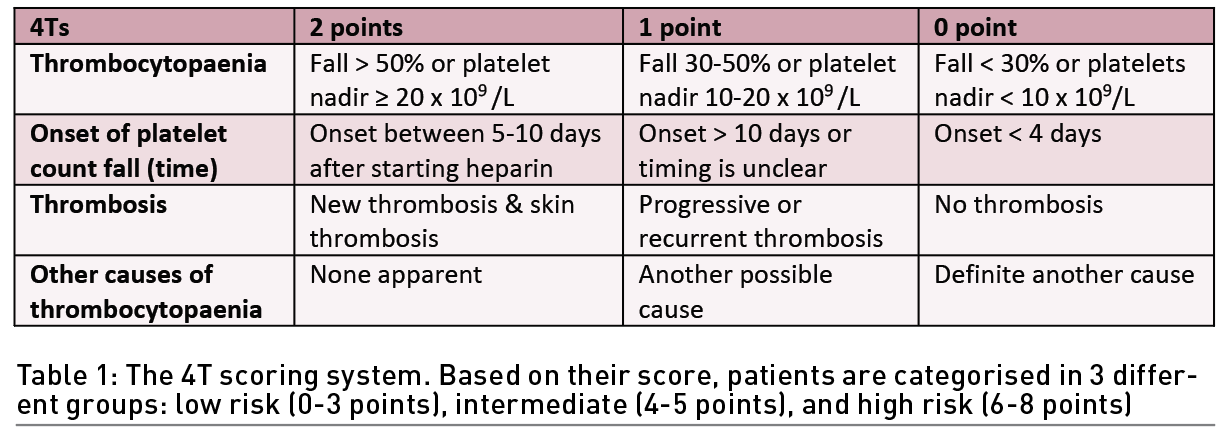
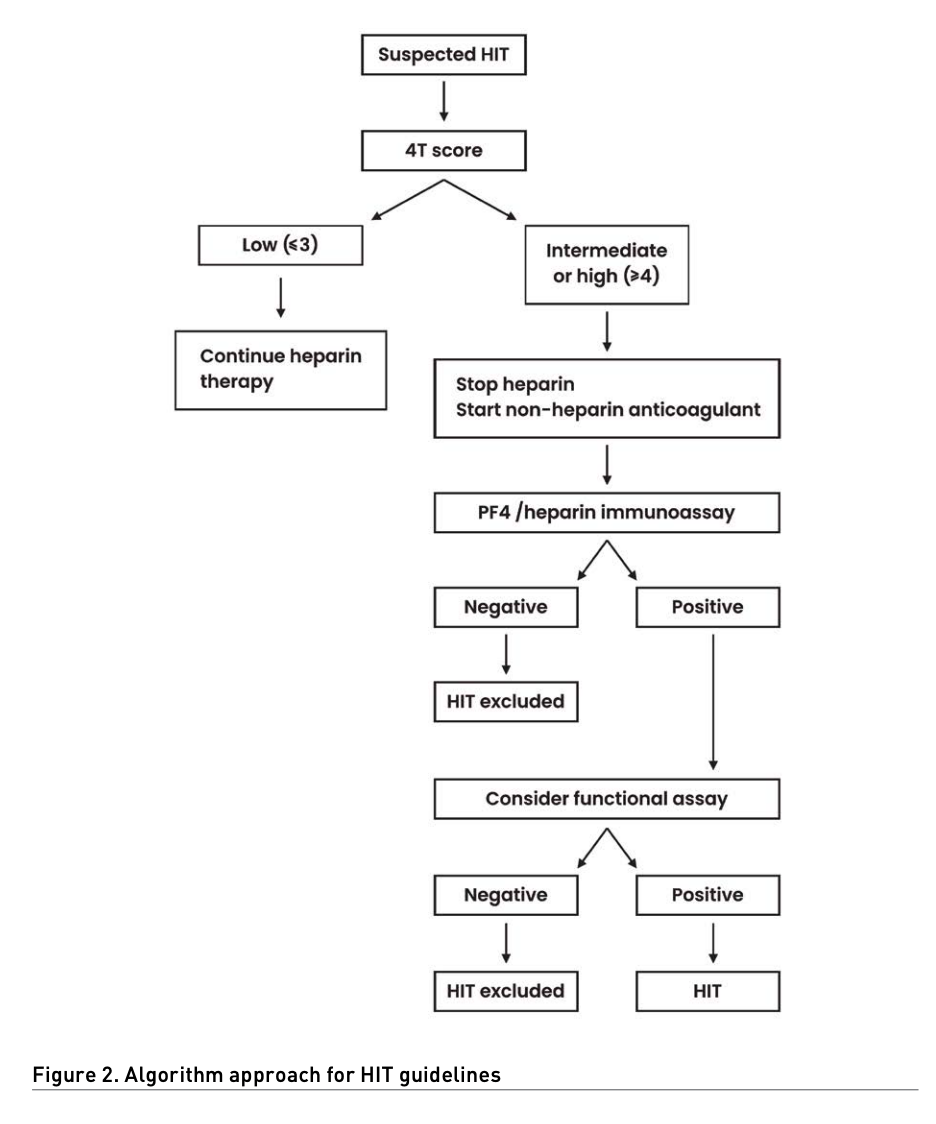
The basic clinical feature of HIT is a platelet count decrease beginning 5-10 days after heparin exposure with or without thrombosis. In patients with suspected HIT, the American Society of Hematology (ASH) guideline panel recommends using the 4Ts score to estimate the probability of HIT (Table 1). The 4Ts score estimates the pretest probability of HIT compared with other causes of thrombocytopaenia while considering 1) platelet count, 2) timing of the onset of the decrease in the patient’s platelet count, 3) thrombosis or other sequelae, and 4) the presence of other causes of thrombocytopaenia. A score from 0 to 2 is given for each category. The maximum score is 8. A score of 6-8 is indicative of a high probability of HIT. A score of 4-5 is indicative of an intermediate presence of HIT, and a score of 0-3 is a low possibility of the presence of HIT. If HIT is suspected because the patient has an intermediate or elevated possibility of HIT based on the 4Ts score (4 to 8 points), the laboratory will assist the physician in making an accurate diagnosis. This can be accomplished by several assays that usually fall into two classes: immunological and functional. For immunological tests, immunoassays (ELISA) are used to detect antibodies against the heparin/PF4 complex and show an excellent negative predictive value but a low positive predictive value. The detection of antiheparin-PF4 antibodies by ELISA does not confirm HIT, but a negative ELISA result makes HIT highly unlikely. If positive, a confirmatory functional assay, which may include the serotonin release assay (SRA) or heparin-induced platelet activation (HIPA), should be ordered, as they have high specificity; however, they require a highly specialised laboratory and are not widely available. In patients with acute HIT without clinically apparent thrombosis, the ASH guideline panel suggests bilateral lower extremity compression ultrasonography to screen for asymptomatic proximal deep venous thrombosis (DVT). If an upper extremity central venous catheter is present, the ASH guideline panel suggests ultrasonography of the limb with the catheter to screen for subclinical DVT.
Differential diagnosis includes infections, drug-induced thrombocytopaenia disseminated intravascular coagulation, liver disease, immune thrombocytopaenia, thrombotic thrombocytopaenic purpura, and haemolytic-uremic syndrome.
Vaccine-induced immune thrombotic thrombocytopaenia (VITT) is a distinct syndrome that consists of an uncommon complication of adenoviral vector COVID-19 vaccines. In VITT, there is a propensity for (1) cerebral and splanchnic vein thrombosis, (2) laboratory features showing consumptive coagulopathy with thrombocytopaenia, low fibrinogen, and elevated D-dimer, and (3) anti-PF4 seropositivity. (Arepally 2021). VITT appears to be similar to heparin-induced thrombocytopaenia (HIT), with both disorders associated with thrombocytopaenia, thrombosis, and the presence of autoantibodies to PF4. Based on the initial reports, female sex and age younger than 60 years were identified as possible risk factors for VITT (Siegler 2021). The mechanism underlying the development of the prothrombotic state and its association with the vaccine are still only partially known because multiple converging prothrombotic pathways may be involved in the pathogenesis (Cines 2021). Treatment consists of therapeutic anticoagulation with non-heparin anticoagulants and prevention of the formation of autoantibody-PF4 complexes, the latter being achieved by the administration of high-dose intravenous immunoglobin (IVIG). Steroids, plasma exchange and monoclonal antibodies may be used.
Management
Cessation of all heparin-containing products and the initiation of alternative non-heparin anticoagulation are the cornerstones of HIT management (Joseph 2019). It is crucial to avoid all heparin-containing products, including catheter locks, heparin flushes, heparin-coated catheters or dialysers, and any heparin-containing medications. LMWH should also be avoided in suspected or confirmed HIT cases, as it cross reacts with HIT antibodies (Davenport 2009). The use of an alternative anticoagulation is mandatory even in those patients with no clinical evidence of thrombosis, as the risk of thrombosis in patients with HIT is high (Warkentin 1996).
The ASH published updated HIT management guidelines in 2018. The initial decision to start non-heparin anticoagulants is guided by the 4Ts score and the risk of bleeding. When confirmatory HIT test results are available, the management plan should be revisited. Regarding the therapeutic agent choice, the ASH suggests the use of argatroban, bivalirudin, danaparoid, fondaparinux, or a direct oral anticoagulant (DOAC) (Cuker 2018). Factors guiding the decision of therapeutic agent choice are mainly related to the drug, the patient, and the centre experience. A meta-analysis found similar rates of platelet recovery, thromboembolic events, haemorrhage, and mortality among the aforementioned anticoagulants (Nilius 2021).
Alternative non-heparin anticoagulation
Argatroban is a synthetic thrombin inhibitor that exerts its anticoagulation effect by reversibly binding thrombin both in the soluble form and within a thrombus. Therefore, in addition to preventing further thrombosis, it also carries an added benefit of targeting older clots. No renal adjustment is required as the drug is hepatically excreted. Moreover, no drug-induced antibodies have been reported with argatroban. No antidote is available; however, aPTT normalises within 2-4 hours of stopping the infusion in patients with no liver impairment. In the absence of liver impairment, the recommended initial infusion dose is 2 mcg/kg/min with dose adjustments according to the aPTT that is checked 2 hours after initiation of the infusion. The target aPTT is 1.5 to 3 times the baseline. The maximum recommended infusion rate is 10 mcg/kg/min. In patients with liver impairment, a lower initial dose is recommended. Activated clotting time (ACT) can also be used to monitor the argatroban anticoagulation effect (the target ACT is 400-600 s).
Bivalirudin is a hirudin analogue and a bivalent direct thrombin inhibitor that acts by binding both the thrombin catalytic site and fibrin binding site. It also leads to the breakdown of von Willebrand factor, thus inhibiting platelet aggregation. It has a short half-life of approximately 25 min. It is primarily enzymatically metabolised and is excreted renally. The initial recommended dose is 0.15-0.2 mg/kg/hr with a target aPTT of 1.5-2.5 times the baseline value (Linkins 2012). Dose reductions are recommended in patients with renal or hepatic impairment (Marcucci 2021). Bivalirudin use is not associated with antibody formation (Warkentin 2004).
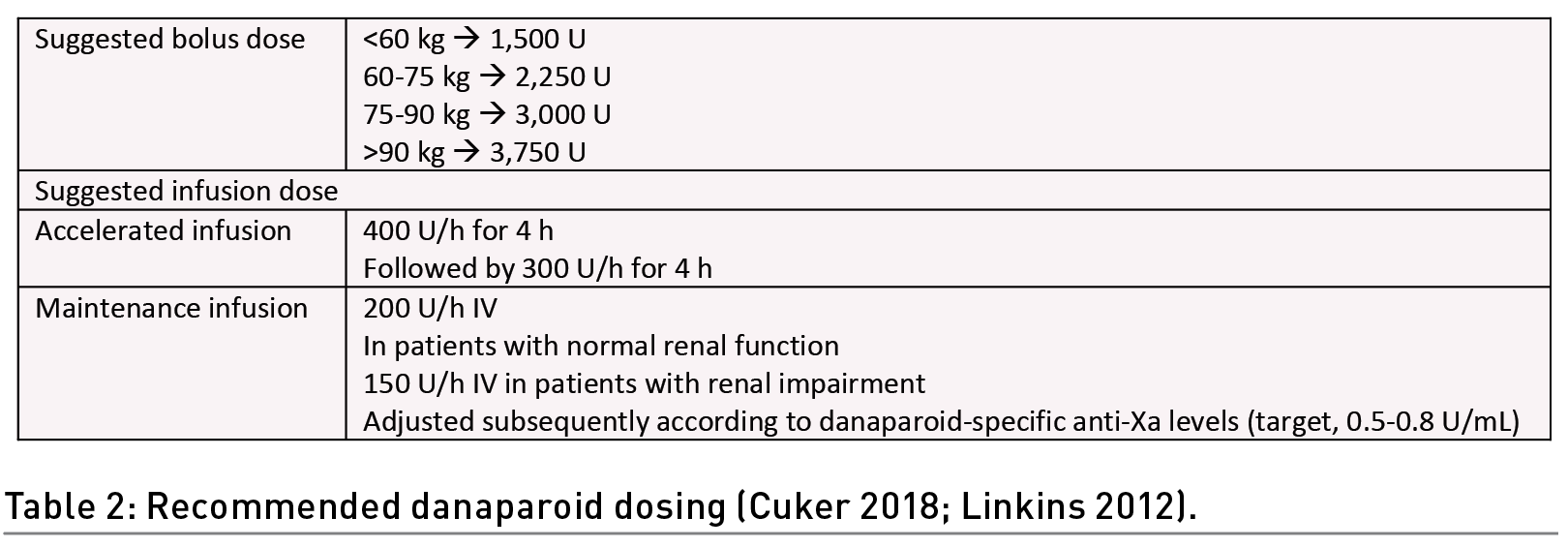
Danaparoid is a low molecular weight heparinoid that consists of a mixture of sulphated glycosaminoglycans isolated from porcine mucosa. It indirectly inhibits both factor Xa and factor II via its inhibition of AT. It also enhances the detachment of PF4 from the platelet surface and disrupts immune complex formation in HIT (Greinacher 2017). The cross-reactivity of danaparoid with PF4 antibodies is low. Its half-life is 24 hours, which may be convenient for dosing but challenging in cases of bleeding. Routine coagulation testing is not needed; however, in cases of renal impairment, anti-factor Xa testing should be performed in addition to dose reduction (Acostamadiedo 2000). The 2012 American College of Chest Physicians and the 2018 ASH guidelines suggest an IV bolus followed by an infusion. The Danaparoid-specific anti-Xa target level is 0.5-0.8 U/mL (Cuker 2018; Linkins 2012) (Table 2).
Fondaparinux is a synthetic pentasaccharide that inactivates factor Xa by selectively and strongly binding AT. Two hours after subcutaneous administration, its bioavailability reaches 100%. It is renally excreted, and its half-life ranges between 17 and 21 hours in healthy volunteers. It can be used for thromboembolic prophylaxis and treatment. It has been studied in obese patients, pregnant patients, cancer-related thrombosis patients, and thrombophilic patients (Nagler 2012). While the drug does not generally react with HIT antibodies in vivo or in vitro, there are rare cases in the literature of HIT antibodies isolated from patients who received fondaparinux. A systematic review that aimed to study the efficacy of using fondaparinux in HIT treatment showed that the risk of thrombosis was similar to that of the approved medications for HIT treatment. As fondaparinux is renally excreted, it should be used with caution in patients with renal impairment (Linkins 2018). Fondaparinux can be self-administered subcutaneously, which decreases hospitalisation length. Transitioning to oral anticoagulation can be performed in the outpatient setting. Additionally, monitoring of the patient’s blood tests are not indicated with fondaparinux, which is different compared to argatroban and bivalirudin. A cost effectiveness analysis showed that fondaparinux is a viable alternative to argatroban and should be considered in the treatment of HIT (Tuleja 2022). The recommended dose of fondaparinux is 5 mg (body weight < 50 kg), 7.5 mg (body weight 50-100 kg) or 10 mg (body weight > 100 kg) by subcutaneous injection once daily.
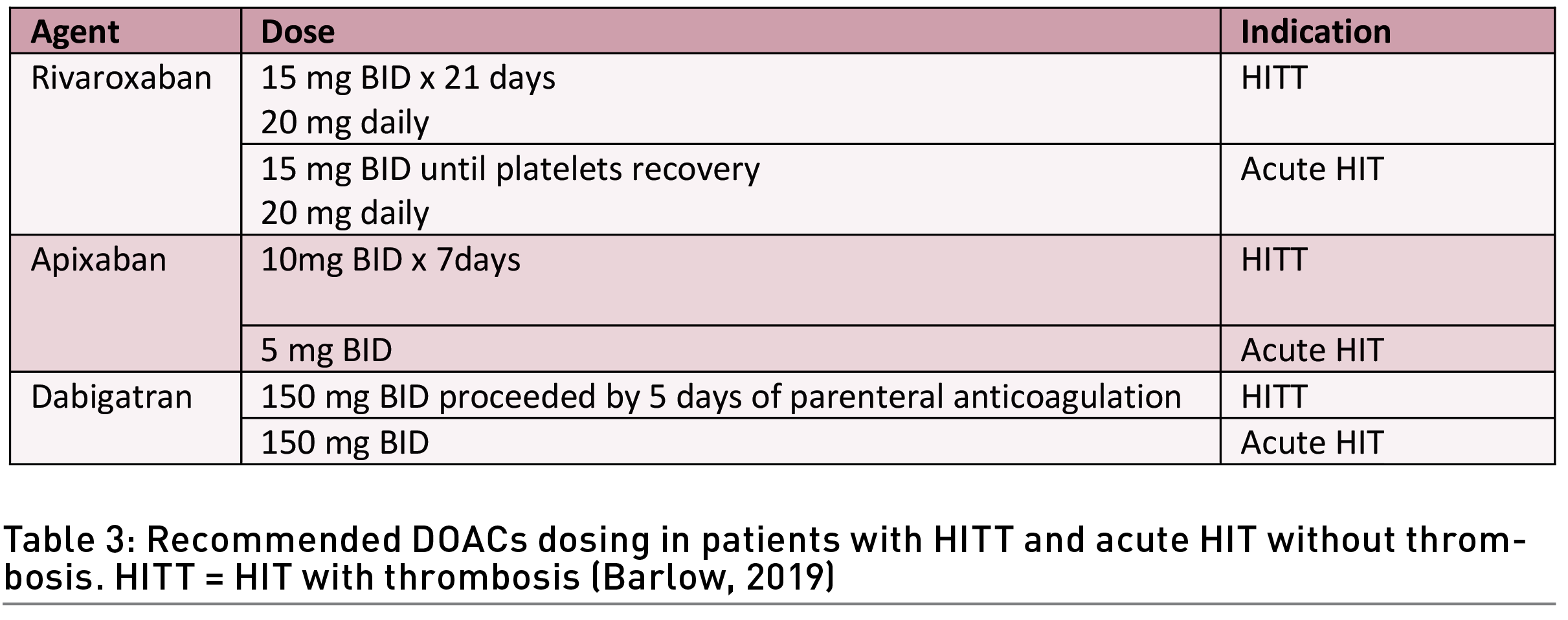
DOACs prevent new and recurrent thrombosis in most cases with minimal bleeding complications (Barlow 2019) (Table 3). Compared to other non-heparin anticoagulants, the advantages of DOAC include oral administration, fixed dosing, and the availability of antidotes (idarucizumab and andexanet alfa).
Traditional treatment is a non-heparin anticoagulant with a transition to vitamin K antagonists (VKA) such as warfarin, upon platelet recovery (platelets > 150 x 109/L). More recently, the ASH guidelines have suggested the use of DOACs over vitamin K antagonists (VKA) (Cuker 2018). Compared to VKA, DOACs carry a decreased risk of bleeding, have fewer drug-drug interactions, require minimal dietary restrictions, allow for the achievement of anticoagulation rapidly, and are not associated with the need for routine laboratory testing. The initiation of warfarin in patients with acute HIT is contraindicated, as warfarin inhibits protein C, leading to a net procoagulant state that can lead to further thrombosis, skin necrosis, and limb gangrene. In patients who are on warfarin prior to HIT diagnosis, reversal of warfarin with vitamin K and the concurrent use of alternative anticoagulation are advised.
Platelet transfusion
Even with profoundly low platelet counts, the risk of bleeding in untreated HIT patients is not high. Previous reports have described thrombosis and delayed platelet recovery in patients with HIT who received platelet transfusions. The ACCP and ASH guidelines recommend against platelet transfusion in patients with an average risk of bleeding. Platelet transfusion may be considered in patients who are actively bleeding, in patients who are at high risk of bleeding, or in preparation for an invasive procedure (Cuker 2018; Linkins 2012).
IVC filter
Based on several studies, the ASH guidelines recommend against using inferior vena cava filters in patients with HIT.
Stepwise Approach
The ASH strongly recommends avoiding the use of alternative non-heparin anticoagulation in patients with suspected HIT and a low 4Ts score. In patients with intermediate- or high-risk 4Ts scores, cessation of heparin and the use of an alternative anticoagulant are recommended initially. In the case of a negative immunoassay, discontinuation of the alternative anticoagulant is recommended, and heparin may be used if still indicated. In patients with high 4Ts scores, additional testing may be indicated. In patients with intermediate or high 4Ts scores and a positive immunoassay, heparin should be avoided, and non-heparin anticoagulation should be continued. When available, a confirmatory activation test is required when the patient has a positive immunoassay test (Figure 2) (Cuker 2018). The duration of anticoagulant use is 4 weeks in patients with isolated HIT and 3 months for HIT with thrombosis.
Conclusion
HIT is a potentially life-threatening complication of heparin exposure. The diagnosis may be challenging and relies on clinical suspicion followed by stepwise testing. It is paramount to stop all forms of heparin if HIT is strongly suspected and to start alternative anticoagulation. Since many centres rely predominantly on an anti-PF4 enzyme-immunoassay (EIA) to diagnose HIT, there is a potential for overdiagnosis, that might lead to increased cost, risk of bleeding, and unnecessarily delayed medical interventions (Warkentin 2011). The optimal management of HIT is based on multidisciplinary collaboration between intensivists and haematologists and the development of protocols.
Conflict of Interest
None.
References:
Acostamadiedo JM, Iyer UG, Owen J (2000) Danaparoid sodium. Expert Opin Pharmacother. 1(4):803-14.
Arepally GM (2017) Heparin-induced thrombocytopenia. Blood. 129(21):2864-2872.
Barlow A, Barlow B, Reinaker T, Harris J (2019) Potential Role of Direct Oral Anticoagulants in the Management of Heparin‐induced Thrombocytopenia. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 39(8):837-853.
Cines DB, Bussel JB (2021) SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med. 384(23):2254-2256.
Cuker A, Arepally GM, Chong BH et al. (2018) American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2(22):3360-3392.
Davenport A (2009) Antibodies to heparin-platelet factor 4 complex: pathogenesis, epidemiology, and management of heparin-induced thrombocytopenia in hemodialysis. Am J Kidney Dis. 54(2):361-74.
Greinacher A, Selleng K, Warkentin TE (2017) Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 15(11):2099-2114.
Jevtic SD, Morris AM, Warkentin TE, Pai M (2021) Heparin-induced thrombocytopenia. CMAJ. 193(20):E736.
Joseph J, Rabbolini D, Enjeti A et al. (2019) Diagnosis and management of heparin‐induced thrombocytopenia: a consensus statement from the Thrombosis and Haemostasis Society of Australia and New Zealand HIT Writing Group. Medical Journal of Australia, 210(11):509-516.
Linkins LA, Dans AL, Moores LK et al. (2012) Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 141(2 Suppl):e495S-e530S.
Linkins L, Hu G, Warkentin T (2018) Systematic review of fondaparinux for heparin-induced thrombocytopenia: When there are no randomized controlled trials. Research And Practice In Thrombosis And Haemostasis. 2(4):678-683.
Marcucci R, Berteotti M, Gori AM et al. (2021) Heparin induced thrombocytopenia: position paper from the Italian Society on Thrombosis and Haemostasis (SISET). Blood Transfus. 19(1):14-23.
Nagler M, Haslauer M, Wuillemin WA (2012) Fondaparinux - data on efficacy and safety in special situations. Thromb Res. 129(4):407-17.
Nilius H, Kaufmann J, Cuker A, Nagler M (2021) Comparative effectiveness and safety of anticoagulants for the treatment of heparin-induced thrombocytopenia. Am J Hematol. 96(7):805-815.
Siegler JE, Cardona P, Arenillas JF et al. (2021) Cerebrovascular events and outcomes in hospitalized patients with COVID-19: The SVIN COVID-19 Multinational Registry. Int J Stroke. 16(4):437-447.
Tuleja A, Salvador D, Muka T et al. (2022) Cost-effectiveness analysis of alternative anticoagulation in suspected heparin-induced thrombocytopenia. Blood Adv. 6(10):3114-3125.
Warkentin TE, Kelton JG (1996) A 14-year study of heparin-induced thrombocytopenia. Am J Med. 101(5):502-7.
Warkentin TE (2004) Bivalent direct thrombin inhibitors: hirudin and bivalirudin. Best Pract Res Clin Haematol. 17(1):105-25.
Warkentin TE (2004) How I diagnose and manage HIT. Hematology Am Soc Hematol Educ Program. 2011:143-9.