ICU Management & Practice, Volume 18 - Issue 1, 2018
The complex nature of the multiple organ dysfunction syndrome (MODS) requires an integrated supportive therapy. Native organs have a continuous crosstalk and have in common in most cases an altered composition of the blood circulating and perfusing them. In this article we describe the concept of extracorporeal organ support (ECOS) for the treatment of combined organ dysfunction in critical illness. ECOS includes all forms of therapies where blood is extracted from the body and processed in different circuits with specific devices and techniques. Simultaneous application of different devices and circuits implies possible interactions among artificial organ support systems with potentially negative consequences. We propose a multidisciplinary effort to combine all these techniques avoiding mistakes and problems and we suggest the creation of a new generation of ECOS equipment with integrated features to avoid artificial organ negative crosstalk.
You might also like: Understanding LVAD & artificial hearts
The management of critically ill patients in the ICU is progressively
increasing in complexity (Kadri et al. 2017). Significant advances in
care, comorbidity and advanced age of patients have led to a greater
severity of illness at admission (Kaukonen et al. 2014). Simultaneous
dysfunction of various organs is frequent, leading to the so-called
multiple organ dysfunction/failure syndrome (MODS/MOFS) (Ziesmann and Marshall 2017).
The complex nature of multiple organ dysfunction syndrome
Several
organ systems are involved in critical illness where initial impairment
of one organ function is often followed by dysfunction or damage in
other organs.This is especially true in the context of sepsis or other
systemic disorders (Ziesmann and Marshall 2017). For example,
the effect of acute kidney injury (AKI) on distant organs is now well
documented (Kellum and Prowle 2018). This phenomenon may be observed with
a primary injury to other single organs followed by secondary dam-
age/dysfunction of other organs (Figure 1). The initial sequence
of events often results in a vicious circle leading to a continuous
negative organ interaction and a progressive worsening of the syndrome
(Husain-Syed et al. 2015). This is typically the case in cardiorenal
syndrome (CRS) where several bidirectional and temporally related
heart–kidney interactions may lead to five different clinical subtypes
(Ronco et al. 2008). The syndrome initiation, the primary organ involved
and the mechanisms are different in nature: haemodynamic alterations
and congestion, iatrogenic effect of interventions, direct toxicity of
drugs or contrast media, neuro-hormonal derangements and
immune-mediated/inflammatory damage (Husain-Syed et al. 2016).
Nevertheless, after a significant organ crosstalk has initiated, the
progressive dysfunction of both organs leads to significant worsening of
the clinical picture.
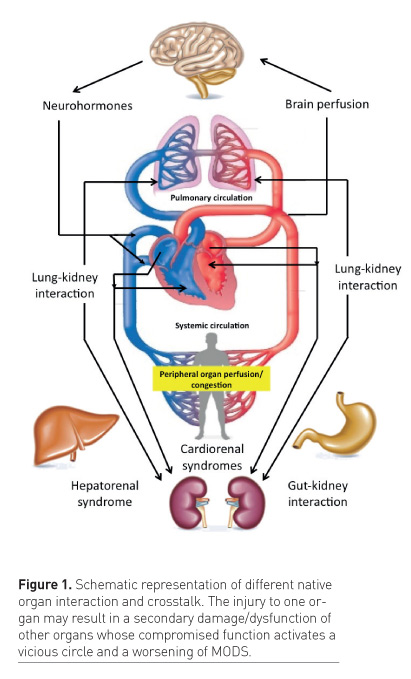
Other conditions may involve acute and chronic lung disease scenarios leading to AKI or accelerated chronic kidney disease (CKD), and vice versa (Husain-Syed et al. 2016).
Some of these interactions include the participation of the heart in an
even more complex cardio-pulmonary-kidney crosstalk (Husain-Syed et al.
2015).
Critically ill patients may develop liver dysfunction in the context of MODS or may suffer from primary liver disorders. Liver dysfunction may become the trigger for several pathological pathways, eventually involving lungs, kidneys and brain (Siddiqui and Stravitz 2014). Combined liver and kidney dysfunction is common and described by different types of hepatorenal syndrome (HRS) (Fukazawa and Lee 2013). AKI represents a well-known complication of liver disease through different biological pathways and it is associated with increased morbidity and mortality. Renal dysfunction in cirrhosis is often functional in nature and secondary to haemodynamic derangements, cardiac dysfunction and altered plasma composition. Nevertheless, an increasing number of patients with cirrhosis may develop structural damage of the kidneys leading to a progressive deterioration of organ function (Arroyo and Jiménez 2000). In turn, once kidney function deteriorates in liver patients, a progressive worsening of the syndrome is typically observed with unfavourable outcomes.
Gut and kidney may also present
reciprocal negative interactions due to primary alterations in host
microbiome profile and disruption of gut barrier function leading to
systemic inflammation, AKI, progression of CKD with effects on uraemic
toxicity and potential increase in cardiovascular risk (Jacobs et al.
2017). On the other hand, the effects of AKI on the increased risk of
bleeding and other derangements of the gastrointestinal tract have been
described (Doi and Rabb 2016).
All these syndromes are often the result
of a mixture of direct organ injury, secondary systemic disorders and
altered tissue perfusion in different organ systems. Preexisting organ
dysfunction can make the clinical picture worse (Rosenthal et al. 2018).
Furthermore, primary and secondary organ injury/dysfunction results
from a complex balance between individual susceptibility and exposure
(insult) intensity (Agarwal et al. 2016).
The interaction between these
two factors is particularly evident in the case of sepsis where several
organs are affected by an exaggerated and uncontrolled imbalance between
the pro- and anti-inflammatory response of the host. The so-called
immune-homeostasis is compromised and organ dysfunction is generally the
result of altered blood perfusion and metabolism at the tissue and
cellular level (Boomer et al. 2011). Although individual characteristics
become less important when the intensity of exposure (insult) is
overwhelming, the contribution of host response to organ injury may
still be significant and precision medicine criteria should be applied
for the final treatment strategy (Zieamann and Marshall 2018).
Multiple organ support therapy (MOST)
Critically ill patients with MODS require
a complex and articulated therapeutic approach that includes
pharmacological strategies (such as antibiotics for infection source
control, circulatory and respiratory support, organ-specific drugs,
correction of abnormalities of coagulation, electrolyte, acid-base,
metabolism) and specific organ support systems. All these interventions
should be integrated in a global strategy to support single organs and
manage the combined effects of multiple organ crosstalk. In a seminal
paper, we described the concept of multiple organ support therapy
(MOST), identifying the possibility to provide simultaneous and combined
support to different failing organ systems (Ronco and Bellomo 2002).
MOST includes oxygenation and ventilatory support (invasive and
noninvasive mechanical ventilation [MV], venovenous extracorporeal
membrane oxygenation [ECMO] and extracorporeal carbon dioxide removal
[ECCO2R]), mechanical circulatory support (intra-aortic balloon pump,
venoarterial ECMO, percutaneous and surgical ventricular assist devices
[VADs] and total artificial heart), renal replacement therapy (RRT) and
extracorporeal liver support (molecular adsorbent recirculating system,
plasmapheresis and sorbent therapies). All these techniques are
currently used in the ICU although very little is known about their
interaction with native organs and other artificial organ support
systems (Ronco 2006).
Extracorporeal organ support (ECOS)
Extracorporeal blood purification
techniques such as haemodialysis or haemofiltration, have been used
successfully for several decades to replace renal function in critically
ill patients with kidney failure. New applications are today emerging
for extracorporeal techniques. The experience with extracorporeal blood
therapies in sepsis suggests redefining the spectrum of application, and
we are today exploring the concept of extracorporeal organ support
(ECOS) to describe all forms of therapies where blood is extracted from
the body and processed in different circuits with specific devices and
techniques (Ranieri et al. 2017). The principle for ECOS is that in MODS
failing organs have in common the blood perfusing their tissues, and
circulating blood becomes the target for specific treatments.
The idea of using extracorporeal
therapies for sepsis came from the occasional observation that septic
patients treated with RRT for AKI displayed a rapid and significant
improvement in haemodynamics, with a reduced requirement of vasopressor
support a few hours after application of the extracorporeal circulation.
Further experiments demonstrated that the ultrafiltrate recovered from
septic patients treated with haemofiltration and injected in healthy
animals produced septic symptoms (Tetta et al. 1998). The improvement in
septic manifestations in patients undergoing RRT suggested a possible
reduction of circulating chemical mediators eliminated in the
ultrafiltrate. The absence of significant variation in circulating
levels of cytokines created some conflicting positions (Sieberth and
Kierdorf 1999) that will never be resolved until a well-designed and
adequately powered trial on extracorporeal therapies in sepsis in the
absence of AKI is performed. Still, the question of whether mortality is
the correct endpoint for such a study is wide open. Nevertheless, some
hypotheses were formulated such as the peak concentration hypothesis
(Honoré et al. 2006), based on the idea that a non-selective elimination
of the peaks of both pro- and anti-inflammatory mediators might
contribute to a restoration of a certain degree of immune-homeostasis
and to a reduction of the severe imbalance produced by the exaggerated
host response to bacterial invasion. Also, a personalised approach,
matching RRT intensity with the risk of albumin and amino acid,
catecholamine and antibiotic loss, should be advocated to avoid
jeopardising the beneficial effects of extracorporeal therapies (Bagshaw
et al. 2016). The culture of this approach comes from the discipline of
nephrology. For years, chronic haemodialysis has sustained thousands of
lives even though a clear understanding of the molecular basis of
uraemia has not been achieved yet. A similar approach can be used for
sepsis and multiple organ failure where altered blood composition
represents the common ground for damage/dysfunction: whatever component
is in excess or defect compared to its physiological concentration in
blood, it can be removed or corrected by a specific extracorporeal
treatment and device. This is the basis for the application of ECOS in
critically ill patients (Figure 2).
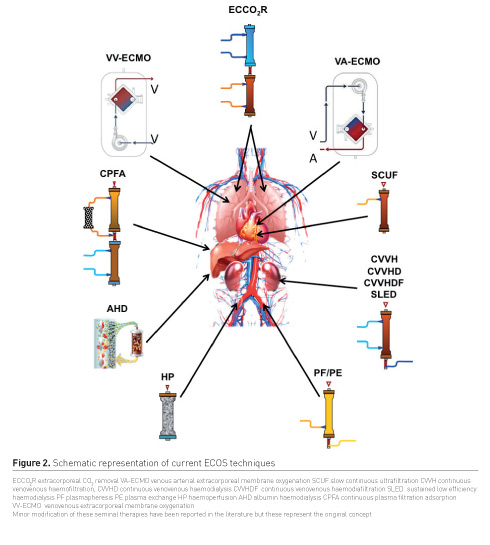
Kidney support
Kidney support can be provided by
different intermittent and continuous blood purification techniques such
as intermittent haemodialysis, slow low-efficiency dialysis, continuous
venovenous haemofiltration, haemodialysis, haemodiafiltration. These
techniques, based on diffusive and/or convective transport of solutes
and water transport by ultrafiltration across a semipermeable membrane,
allow adequate blood purification, acid-base/electrolyte correction and
volume control. In the case of sepsis, such techniques are further
expanded with the use of high-volume haemofiltration (HVHF), coupled
plasma filtration adsorption (CPFA) and high-cut-off membrane (HCO)
applications. The last two techniques are also used when liver
dysfunction or rhabdomyolysis are present and large molecular size
molecules are to be removed from the circulation. In the case of
protein-bound solutes, albumin dialysis (AHD) has also been suggested as
well as plasmapheresis (PP) or plasma exchange (PE). A special
nomenclature has been created to better define the characteristics of
each component of the extracorporeal circuit (Neri et al. 2016) and each
specific technique (Villa et al. 2016).
Adsorption
Adsorption has been proposed as a third
mechanism for solute removal from the circulation. Sorbents prepared in
specific cartridges can be placed in direct contact with blood as in the
case of direct haemoperfusion (HP), or used after plasma filtration
(PF) from whole blood (PFAD) to avoid direct contact of platelets and
white cells with the sorbent particles. After plasma is processed in the
sorbent bed, blood is reconstituted and returned to the patient. HP has
been used for years in case of acute intoxication, hyperbilirubinaemia
and immunoadsorption. Recently, biocompatible sorbent devices have been
created for endotoxin removal (polymyxin-B haemoperfusion [PMX-HP -
Toray Medical Company]) or cytokine removal (cartridges for HP from
Cytosorbents Corporation, Jafron Biomedical or others) in severe sepsis
or septic shock.
Heart support
Heart support has been originally
achieved removing the excess of fluid in the body by ultrafiltration
when diuretics cannot provide adequate diuresis. The spectrum of
extracorporeal techniques has today expanded to other options.
Venoarterial (VA-ECMO) is used in patients with acute cardiac or
circulatory failure to restore end-organ perfusion and organ function,
and to bridge either to recovery, to definite cardiac support (e.g.
ventricular assist devices, VADs) or heart transplantation. MODS is
particularly common in patients requiring cardiac support and use of
lung support and RRT may become additionally necessary (Van Dorn et al.
2018). Again VA-ECMO is part of ECOS because blood is processed outside
the body while VAD, Total implantable heart, Impella® (Abiomed) or
intra-aortic balloon pumps technologies belong to the MOST category but
not to ECOS.
Lung support
Lung support in the context of ECOS has
been traditionally identified with venovenous (VV)-ECMO. VV-ECMO is
mostly used for correction of hypoxaemia refractory to lung-protective
ventilation and prone position in patients with severe acute respiratory
distress syndrome. The experience coming again from haemodialysis
brought into clinical practice however the possibility to achieve
partial lung support with a certain removal of CO2 from the circulation.
This concept of “respiratory dialysis” has further evolved to a system
where a small oxygenator is placed in series with a CVVH circuit
(Romagnoli et al. 2016). The technique called ECCO2R is used as an
alternative or supplement to mechanical ventilation for correction of
hypercapnia, but not for blood oxygenation since the blood flows through
the circuit are relatively low (350-450 ml/min). Recently, RRT in
conjunction with ECCO2R has been advocated to allow “super-protective”
MV settings, and reduction of vasopressor demands in patients with ARDS
experiencing AKI (Allardet-Servent et al. 2015). In some cases, ECCO2R
can also allow continuation of noninvasive MV, thus avoiding invasive
MV.
Liver support
Liver support can be provided by albumin
dialysis, plasma filtration/adsorption, plasma exchange and
haemoperfusion. Not only removal of bilirubin and other protein-bound
toxins can be achieved by these techniques, but also significant
reduction of ammonium level can be observed during treatment. The
Molecular Adsorbent Recirculating System MARS® (Gambro®), Prometheus®
therapy system (Fresenius Medical) and other equipment based on cascade
filtration and dialysis with albumin-based dialysate and sorbents are
today available for this purpose (Faybik and Krenn 2013).
Native and artificial organ crosstalk
There is a clear need to explore
crosstalk and interactions between different organ systems in the
critically ill patient. The literature on complex syndromes with
multiorgan involvement emphasises the need for multidisciplinary
management. In these conditions, the level of multiple organ dysfunction
makes MOST highly recommended or even mandatory. Frequently however,
patients who display clear indication for ECMO and are undergoing such a
complex therapy, may require further organ support with the addition of
RRT, liver support, haemoperfusion for detoxification, or cardiac
support. In these circumstances, extracorporeal support and organ
replacement may become safer and more uniform if different functions are
combined in a fully integrated hardware. Fluid balance, solute removal,
CO2 removal, aromatic amino acid removal, electrolyte and acid base
equilibration, blood detoxification and oxygenation should be considered
a continuum, where the artificial organ crosstalk is constant.
Variations in CO2 must consider the use of buffers in dialysis or the
application of citrate as anticoagulant for an adequate equilibrium of
acid-base. The future is likely to see the introduction of a unified
hardware with special circuitry that will allow performance of all
different organ support therapies on demand, simply escalating or
de-escalating the complexity of the system. Thus, from ECMO and RRT, a
patient may be progressively moved to ECCO2R and intermittent
haemodialysis and, finally, even be discharged with organ support
including chronic haemodialysis and respiratory dialysis in case of
non-recovery or progression towards chronic illness.
Next generation ECOS equipment
If MOST is applied and especially in the
context of multipurpose ECOS, artificial organ crosstalk should be
considered by a multidisciplinary task force to avoid negative
interactions and unwanted side effects. An integrated monitoring of
patients, chemistry and machine parameters will offer the basis for
“smart” biofeedback leading to correction in prescription and delivery
of extracorporeal organ support (Ricci et al. 2017).
We strongly advocate the need for next generation ECOS machines to achieve harmonisation of components, techniques and operations of multiple extracorporeal therapies. We suggest the possibility to perform simultaneous multiple functions and techniques optimising artificial organ crosstalk while avoiding unwanted side effects or operational drawbacks due to poor integration of prescription and delivery parameters. Further studies are needed to establish the ideal timing of interventions, to find out whether early implementation impacts organ recovery and optimises resource utilisation, and to identify the patient groups that can be expected to benefit from long-term organ support.
Conflict of interest
Claudio Ronco declares he has no conflict
of interest. Zaccaria Ricci declares he has no conflict of interest.
Faeq Husain-Syed declares he has no conflict of interest.
Abbreviations
AHD albumin dialysis
AKI acute kidney injury
CKD chronic kidney disease
CPFA coupled plasma filtration adsorption
ECCO2R extracorporeal carbon dioxide removal
ECMO extracorporeal membrane oxygenation
ECOS extracorporeal organ support
HCO high-cut-off membrane
HP haemoperfusion
HRS hepatorenal syndrome
HVHF high volume haemofiltration
MODS/MOFS Multiple Organ Dysfunction/Failure Syndrome
MOST multiple organ support therapy
MV mechanical ventilation
PE plasma exchange
PF plasma filtration
PFAD plasma filtration adsorption dialysis
PP plasmapheresis
RRT renal replacement therapy
VAD ventricular assist device
References:
Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014 Apr 2;311(13):1308-16
Ziesmann MT, Marshall JC. Multiple Organ Dysfunction: The Defining Syndrome of Sepsis. Surg Infect (Larchmt). 2018 Jan 23. doi: 10.1089/sur.2017.298.
Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018 Jan 22. doi: 10.1038/nrneph.2017.184.
Husain-Syed F, McCullough PA, Birk HW, Renker M, Brocca A, Seeger W, Ronco C. Cardio-Pulmonary-Renal Interactions: A Multidisciplinary Approach. J Am Coll Cardiol. 2015 Jun 9;65(22):2433-48.
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008 Nov 4;52(19):1527-39.
McCullough PA, Kellum JA, Haase M, Müller C, Damman K, Murray PT, Cruz D, House AA, Schmidt-Ott KM, Vescovo G, Bagshaw SM, Hoste EA, Briguori C, Braam B, Chawla LS, Costanzo MR, Tumlin JA, Herzog CA, Mehta RL, Rabb H, Shaw AD, Singbartl K, Ronco C. Pathophysiology of the cardiorenal syndromes: executive summary from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:82-98
Husain-Syed F, Slutsky AS, Ronco C. Lung-Kidney Cross-Talk in the Critically Ill Patient. Am J Respir Crit Care Med. 2016 Aug 15;194(4):402-14. doi: 10.1164/rccm.201602-0420CP. Review. PubMed PMID: 27337068.
Siddiqui MS, Stravitz RT (2014) Intensive Care Unit Management of Patients with Liver Failure. Clinics in Liver Disease 18:957-978.
Fukazawa K, Lee HT. Updates on Hepato-Renal Syndrome. J Anesth Clin Res. 2013 Sep 27;4(9):352.
Arroyo V, Jiménez W. Complications of cirrhosis. II. Renal and circulatory dysfunction. Lights and shadows in an important clinical problem. J Hepatol. 2000;32(1 Suppl):157-70
Jacobs MC1, Haak BW, Hugenholtz F, Wiersinga WJ. Gut microbiota and host defense in critical illness. Curr Opin Crit Care. 2017 Aug;23(4):257-263.
Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int. 2016 Mar;89(3):555-64.
Rosenthal MD, Kamel AY, Rosenthal CM, Brakenridge S, Croft CA, Moore FA. Chronic Critical Illness: Application of What We Know. Nutr Clin Pract. 2018 Feb;33(1):39-45.
Agarwal A, Dong Z, Harris R, Murray P, Parikh SM, Rosner MH, Kellum JA, Ronco C; Acute Dialysis Quality Initiative XIII Working Group. Cellular and Molecular Mechanisms of AKI. J Am Soc Nephrol. 2016 May;27(5):1288-99.
Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD 2nd, Kreisel D, Krupnick AS, Srivastava A, Swanson PE, Green JM, Hotchkiss RS. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011 Dec 21;306(23):2594-605.
Ronco C, Bellomo R. Acute renal failure and multiple organ dysfunction in the ICU: from renal replacement therapy (RRT) to multiple organ support therapy (MOST). Int J Artif Organs. 2002 Aug;25(8):733-47.
Ronco C. Recent evolution of renal replacement therapy in the critically ill patient. Crit Care. 2006 Feb;10(1):123
Ranieri VM, Brodie D, Vincent JL. Extracorporeal organ support: from technological tool to clinical strategy supporting severe organ failure. JAMA 2017; 26;318(12).
Tetta C, Cavaillon JM, Schulze M, Ronco C, Ghezzi PM, Camussi G, Serra AM, Curti F, Lonnemann G. Removal of cytokines and activated complement components in an experimental model of continuous plasma filtration coupled with sorbent adsorption. Nephrol Dial Transplant. 1998 Jun;13(6):1458-64.
Sieberth HG, Kierdorf HP. Is cytokine removal by continuous hemofiltration feasible? Kidney Int Suppl. 1999 Nov;(72):S79-83
Honore' PM, Joannes-Boyau O, Merson L, Boer W, Piette V, Galloy AC, Janvier G. The big bang of hemofiltration: the beginning of a new era in the third millennium for extra-corporeal blood purification! Int J Artif Organs. 2006 Jul;29(7):649-59.
Bagshaw SM, Chakravarthi MR, Ricci Z, Tolwani A, Neri M, De Rosa S, Kellum JA, Ronco C; ADQI Consensus Group. Precision Continuous Renal Replacement Therapy and Solute Control. Blood Purif. 2016;42(3):238-47.
Neri M, Villa, G, Garzotto F, et al. Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: basic principles. Crit Care 2016; 10;20(1):318
Villa G, Neri M, Bellomo R, et al. Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: practical considerations. Crit Care 2016; 10;20(1):283
Van Dorn CS, Aganga DO, Johnson JN. Extracorporeal membrane oxygenation, Berlin, and ventricular assist devices: a primer for the cardiologist. Curr Opin Cardiol. 2018 Jan;33(1):87-94.
Romagnoli S, Ricci Z, Ronco C. Novel Extracorporeal Therapies for Combined Renal-Pulmonary Dysfunction. Semin Nephrol. 2016 Jan;36(1):71-7.
Allardet-Servent J, Castanier M, Signouret T, Soundaravelou R, Lepidi A, Seghboyan JM. Safety and Efficacy of Combined Extracorporeal CO2 Removal and Renal Replacement Therapy in Patients With
Acute Respiratory Distress Syndrome and Acute Kidney Injury: The Pulmonary and Renal Support in Acute Respiratory Distress Syndrome Study. Crit Care Med 2015; 43: 2570–81
Faybik P, Krenn CG. Extracorporeal liver support. Curr Opin Crit Care. 2013 Apr;19(2):149-53.
Ricci Z, Romagnoli S, Ronco C (2017) Automatic Dialysis and Continuous Renal Replacement Therapy: Keeping the Primacy of Human Consciousness and Fighting the Dark Side of Technology. Blood Purif. 2017 Oct 21;44(4):271-275.









