Abstract
The development of digital breast tomosynthesis (DBT) for the detection of breast cancer has resulted in many trials showing that an improvement in detection is possible with DBT. However, these trials have also shown that reading DBT images is considerably slower than reading standard digital mammography (DM) cases. Not surprisingly, it takes longer to read a stack of 50 image slices than one standard mammogram. This increase in reading time for DBT interpretation limits its introduction in large screening programs, such as the regional or national screening programs commonly found in Europe. Therefore, for the better part of this decade, multiple efforts and research have taken place to demonstrate the feasibility of different time-saving strategies when reading DBT exams. As a result, it now seems more feasible than ever that the reading time in DBT could be reduced to match or even be lower than that of DM. For these strategies to be introduced in every-day use, however, some additional studies are needed. Here, we review the strategies proposed up to now to reduce the time required for interpretation of DBT cases in breast cancer screening, and discuss the current limitations in knowledge regarding some of these interpretations.
Introduction
Since digital mammography (DM) is a two-dimensional imaging modality, mammograms of the three-dimensional breast suffer from the phenomenon of tissue superposition. That is, tissues that are separated only vertically in the breast during compression are projected to the same location in the mammogram. This can result in either normal tissues resembling a malignant finding, lowering specificity, or normal tissue masking a real finding, lowering sensitivity. Of course, the higher the proportion of the breast that is composed of dense fibroglandular tissue, the higher the risk of superposition. To ameliorate this effect, currently a mammographic examination, especially for screening for breast cancer, involves the acquisition of two views; the cranio-caudal (CC) and the medio-lateral oblique (MLO) views. However, this is not a perfect solution, since the loss of performance due to this effect, especially in dense breasts, persists.
Digital breast tomosynthesis (DBT) was introduced mostly to reduce this problem of tissue superposition. DBT involves the acquisition of multiple low-dose mammography-like projections from various angles over a limited angular range around the compressed breast (Fig. 1). These projections are then used to reconstruct a pseudo-3D volume depicting the breast tissue distribution [1–3]. This pseudo-3D volume is enough to reduce the impact of tissue superposition despite limited vertical spatial resolution, and results in improved clinical performance compared to DM [4–10].
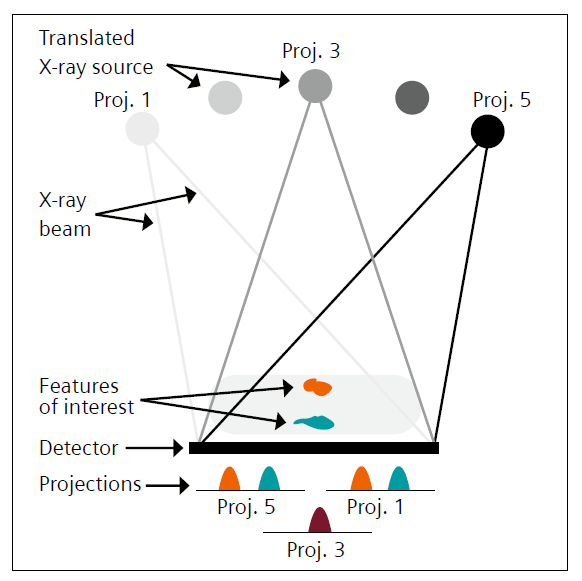
1 Schematic of a digital breast tomosynthesis acquisition, showing a geometry equal to that used in mammography, but with the X-ray source rotating around the compressed breast, acquiring a projection image at each position. The changing X-ray source position results in different projection images, with the features in the breast changing location in the images depending on their vertical location.
However,
a single DBT image typically consists of a stack of ~50 slices for a breast
with a typical thickness under compression of about 50 mm. This increases the
amount of information generated by DBT to be reviewed by the interpreting
radiologist, which results in a reading time that has been repeatedly reported
to be double that of DM [11]. This increase in the demand of radiologist
resources is one of the most important challenges needing to be overcome before
DBT could be introduced in large population screening programs as a replacement
of DM. However, several alternative acquisition and reading strategies may be
useful in optimizing the interpretation of DBT-based screening. This would
allow for the potential of DBT, and its promise of improved outcomes, to
finally be introduced in high-volume screening programs without a substantial increase
in the expenditure of healthcare resources. The strategies and alternatives
that have been proposed or are being investigated can be divided into two
categories: alternative strategies to reduce the number of images that need to
be interpreted, and strategies to read DBT faster.
Reduction of images to be read
As mentioned, currently a screening DM examination consists of the acquisition of two views per breast. The main reason for this is the attempt to ameliorate the effects of tissue superposition. Since this effect is, to a great extent, solved by DBT, then perhaps it is feasible to not acquire two views of each breast, and therefore, only acquire MLO DBT views during screening. If this were the case, the MLO view would be the chosen one due to it being the view with the largest tissue coverage.
The Malmö Breast Tomosynthesis Screening Trial (MBTST) involved the comparison of the screening performance of such a DBT acquisition strategy (MLO view-only), to that of two-view DM [5, 6]. In this prospective screening trial involving almost 15,000 cases, the use of single-view DBT resulted in an increase in the cancer detection rate of 34% over that obtained in the two-view DM arm; from 6.5 to 8.7 cancers per 1,000 women screened [6]. This strategy also resulted in an important increase in the recall rate of 44% (from 2.5% to 3.6%). However, the baseline recall rate was very low to begin with, and the DBT review did not include the use of prior images, an effective tool that is known to reduce recall rate substantially [12]. In a retrospective observer study, Rodriguez Ruiz et al. compared the detection performance resulting from interpreting single-view DBT to that of single-view DBT + single-view DM, two-view DBT + twoview DM, and two-view DM only [13]. Although the retrospective, enriched case set nature of this study of course involved fewer cases than that in the MBTST, this trial allowed for the evaluation of multiple acquisition strategies, with all cases of all strategies interpreted by all participating radiologists. The authors did not detect any difference in performance among the four acquisition strategies. Therefore, it seems feasible that single-view DBT could be used for screening for breast cancer. However, both of these studies used the same wide-angle DBT system. Therefore, the generalizability of these results to DBT imaging performed with narrower-angle systems remains to be evaluated.
In European population screening programs, the most common standard is that all cases are double read by two different breast radiologists. Two other prospective trials, as part of their investigation into screening DBT, tested the hypothesis that the reduction in superposition effects with DBT results in images being easier to interpret, and therefore double reading not yielding as large an improvement as with DM. In the STORM trial, Houssami et al. determined that single-reading of DM+DBT still resulted in an increase of over 40% in the cancer detection rate and a 26% reduction in recall rate, compared to double-reading DM alone [14]. An important improvement in performance was also detected by Romero Martin et al. as part of the prospective DBT trial in Cordoba, Spain [15]. In that study, the increase in the cancer detection rate with single-reading of DBT with a synthetic mammogram (a mammogram-like image generated from the DBT data) was over 20% compared to that with DM alone, while recall rate was reduced by over 40%.
With the introduction of AI-based automated systems that seem to be approaching, if not already have matched, human performance in interpreting breast images, both DM and DBT (16,17), it is now feasible to think that an AI system could be used to interpret all images, and that only the ones picked out as being more suspicious would need to be reviewed by a breast radiologist. This concept of triaging of normal cases has been investigated by a number of different research groups, all, for now, on DM images, having found that an important reduction in caseload can be achieved (ranging from 20% to 90%), with no loss in overall performance [18–20]. Given the similarity in the results of studies that have compared the stand-alone performance of such AI systems for DM and DBT, it could be expected that the same performance when using these systems for triaging of normal cases would be achievable. However, before such triaging could be introduced in the screening realm, its impact on large-scale screening programs would have to be evaluated prospectively with real screening prevalence. This is especially important since it could be expected that the radiologists‘ behavior will be affected when facing a case set that has been through triaging by an AI system. Therefore, prospective clinical trials that gauge this impact are necessary.
Faster reading of images
The three strategies discussed above aim to reduce the number of images that are acquired or need interpretation by a breast radiologist. Once this number has been optimized, it would be beneficial to also minimize the time spent in interpreting each of these images. For this, two strategies have been proposed, the use of slabbing, and the synthetic image-driven interpretation of the case.
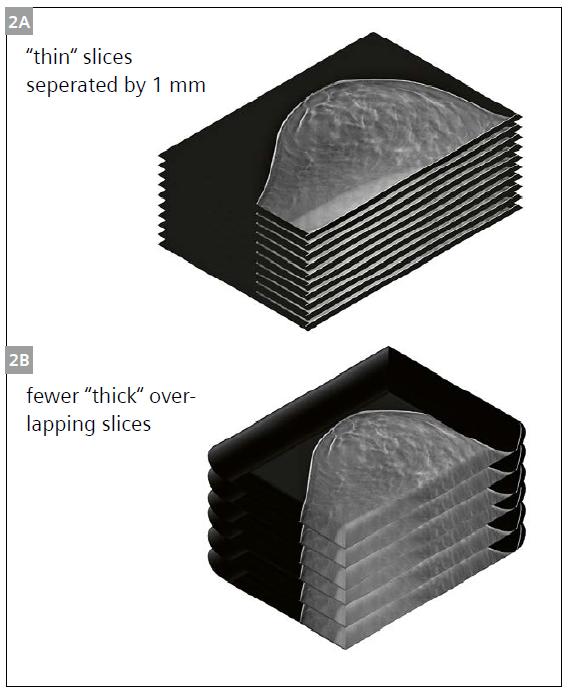
To understand the motivation for presenting the reconstructed DBT volume as a few slabs instead of many thin slices, it should be noted that the spatial resolution in the vertical direction in DBT is very poor. Given the narrow angles subtended during a complete DBT acquisition (the tube movement of the widest angle DBT system covers an angular range of 50°), the DBT “volume“ is actually composed of highly non-isotropic voxels. The signals in the vertical direction included in one voxel can be considerably farther away than the 1 mm often mis-quoted as the slice thickness. In DBT, the slices are reconstructed 1 mm apart from each other, but this does not mean that they are 1 mm thick. In fact, information from 5 mm or more away from the center of the slice may be included in a DBT slice [21]. Therefore, it could be logical that instead of dividing up a typical 50 mm thick breast into fifty 1 mm slices, such a breast could be depicted with considerably fewer, but thicker, slabs. Interpretation of these thicker slabs could be expected to take less time than interpretation of many more thinner slices.
However, it should be ensured that all data required to produce a tomosynthesis volume is available in post-processing, meaning the reader can still choose to see the 1mm slices after scrolling through the 8 mm slabs. In a pair of studies evaluating this hypothesis, it was found that using 2 mm thick slabs resulted in a reduction of 20% in the reading time, while still rendering all lesions visible [22, 23]. In another study, Agasthya et al. compared the reading time and performance when radiologists interpreted 8 mm slabs that overlapped by 3 mm to that of interpreting the regular slices (Fig. 2) [24]. The use of the slabbing technique resulted in equal detection performance with a 30% reduction in the reading time.
Another reading strategy that could substantially reduce the reading time per image is using the synthetic mammogram as the primary image for detection, instead of the reconstructed DBT stack. Under such a scenario, the DBT stack would not be reviewed by the radiologist to detect suspicious findings. Rather, the interpreting radiologist would review the synthetic mammogram, and, if any suspicious area is detected, he/she would, if needed, review that area in the DBT stack to determine if that is, indeed, a finding that needs to be recalled, or an innocuous consequence of tissue superposition or other effect on the synthetic mammogram. An early study evaluating the feasibility of such an approach was performed by Murphy et al., finding that although 13% of the cancers included in the study would have been downgraded in suspicion, they still warranted recall, and therefore they would not have been missed [25]. It should be pointed out, however, that this is, as of now, not yet the intended use of the synthetic mammogram, and there are still probably many improvements that are needed in the generation of these images before they can be reliably used as the primary source for detection of actionable findings. However, with the advent of improved algorithms for constructing these synthetic images, probably in the future with AI having a role in this aspect, it can be expected that this could be a viable strategy in the future, especially for the interpretation of “easier“ cases.
Conclusion
It can be expected that all or a combination of these time-saving strategies, be they to acquire fewer images, have fewer images be interpreted by breast radiologists thanks to their interpretation by stand-alone AI systems, and/or by reading each image faster, could result in DBT based screening requiring the same, or fewer, resources as current DM-based screening, while resulting in improved lesion detection performance. For some of these strategies there is still a lot of evidence that needs to be gathered, or algorithms that need improvement, although some of them seem to be closer to implementation. In either case, demands on breast radiologists to reduce the time to make DBT screening in large population programs a reality seems feasible, soon.
- Niklason LT, Christian BT, Niklason LE, Kopans DB, Castleberry DE, Opsahl-Ong BH, et al. Digital tomosynthesis in breast imaging. Radiology. 1997 Nov;205(2):399–406.
- Sechopoulos I. A review of breast tomosynthesis. Part I. The image acquisition process. Med Phys. 2013 Jan;40(1).
- Sechopoulos I. A review of breast tomosynthesis. Part II. Image reconstruction, processing and analysis, and advanced applications. Med Phys. 2013 Jan;40(1).
- Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast Cancer Screening Using Tomosynthesis in Combination With Digital Mammography. JAMA. 2014 Jun 25;311(24):2499.
- Lång K, Andersson I, Rosso A, Tingberg A, Timberg P, Zackrisson S. Performance of one-view breast tomosynthesis as a stand-alone breast cancer screening modality: results from the Malmö Breast Tomosynthesis Screening Trial, a population-based study. Eur Radiol. 2016;26(1):184–90.
- Zackrisson S, Lång K, Rosso A, Johnson K, Dustler M, Förnvik D, et al. One-view breast tomosynthesis versus two-view mammography in the Malmö Breast Tomosynthesis Screening Trial (MBTST): a prospective, population-based, diagnostic accuracy study. The Lancet Oncology. 2018 Nov;19(11):1493–503.
- Gilbert F, Tucker L, Gillan M, Willsher P, Cooke J, Duncan K, et al. The TOMMY trial: a comparison of TOMosynthesis with digital MammographY in the UK NHS Breast Screening Programme. Health Technol Assess. 2015 Jan 1;19(4).
- Skaane P, Bandos AI, Niklason LT, Sebuødegård S, Østerås BH, Gullien R, et al. Digital Mammography versus Digital Mammography Plus Tomosynthesis in Breast Cancer Screening: The Oslo Tomosynthesis Screening Trial. Radiology. 2019 Feb 19;182394.
- Bernardi D, Macaskill P, Pellegrini M, Valentini M, Fantò C, Ostillio L, et al. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): a population-based prospective study. The Lancet Oncology. 2016;17(8):1105–13.
- Houssami N, Skaane P. Overview of the evidence on digital breast tomosynthesis in breast cancer detection. The Breast. 2013;22(2):101–8.
- Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Prospective trial comparing full-field digital mammography (FFDM) versus combined FFDM and tomosynthesis in a population-based screening programme using independent double reading with arbitration. Eur Radiol. 2013 Aug;23(8):2061–71.
- Roelofs AAJ, Karssemeijer N, Wedekind N, Beck C, van Woudenberg S, Snoeren PR, et al. Importance of Comparison of Current and Prior Mammograms in Breast Cancer Screening. Radiology. 2007 Jan 1;242(1):70–7.
- Rodriguez-Ruiz A, Gubern-Merida A, Imhof-Tas M, Lardenoije S, Wanders A, Andersson I, et al. One-View Digital Breast Tomosynthesis as a Stand-Alone Modality for Breast Cancer Detection: Do We Need More? Eur Radiol. 2018;28(5):1938–48.
- Houssami N, Macaskill P, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Breast screening using 2D-mammography or integrating digital breast tomosynthesis (3D-mammography) for single-reading or double-reading – Evidence to guide future screening strategies. European Journal of Cancer. 2014 Jul;50(10):1799–807.
- Romero Martin S, Raya Povedano JL, Cara Garcia M, Santos Romero AL, Pedrosa Garriguet M, Alvarez Benito M. Prospective study aiming to compare 2D mammography and tomosynthesis + synthesized mammography in terms of cancer detection and recall. From double reading of 2D mammography to single reading of tomosynthesis. Eur Radiol. 2018 Jan 2; 28(6):2484-91.y
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, Broeders M, Gennaro G, Clauser P, et al. Stand-Alone Artificial Intelligence for Breast Cancer Detection in Mammography: Comparison With 101 Radiologists. J Natl Cancer Inst. 2019 Sep 1;111(9):916–22.
- Conant EF, Toledano AY, Periaswamy S, Fotin SV, Go J, Boatsman JE, et al. Improving Accuracy and Efficiency with Concurrent Use of Artificial Intelligence for Digital Breast Tomosynthesis. Radiology: Artificial Intelligence. 2019 Jul 1;1(4):e180096.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, Teuwen J, Broeders M, Gennaro G, et al. Can we reduce the workload of mammographic screening by automatic identification of normal exams with artificial intelligence? A feasibility study. Eur Radiol. 2019 Sep 1;29(9):4825–32.
- Yala A, Schuster T, Miles R, Barzilay R, Lehman C. A Deep Learning Model to Triage Screening Mammograms: A Simulation Study. Radiology. 2019 Aug 6;293(1):38–46.
- Kyono T, Gilbert FJ, van der Schaar M. Improving Workflow Efficiency for Mammography Using Machine Learning. Journal of the American College of Radiology. 2019 May 30 17(1PA):56-63.
- Rodríguez-Ruiz A, Castillo M, Garayoa J, Chevalier M. Evaluation of the technical performance of three different commercial digital breast tomosynthesis systems in the clinical environment. Physica Medica. 2016 Jun 1;32(6):767–77.
- Dustler M, Andersson M, Förnvik D, Timberg P, Tingberg A. A study of the feasibility of using slabbing to reduce tomosynthesis review time. Proc SPIE. 2013;8673:86731L-86731L – 6.
- Petersson H, Dustler M, Tingberg A, Timberg P. Evaluation of the possibility to use thick slabs of reconstructed outer breast tomosynthesis slice images. Proc of SPIE 2016 ;9787:97871M.
- Agasthya GA, D‘Orsi CJ, Holbrook A, Ho C, Piraner M, Newell M, et al. Reduction in digital breast tomosynthesis interpretation time by slabbing of the reconstructed slices. In: European Congress of Radiology. Vienna, Austria; 2016.
- Murphy MC, Coffey L, O‘Neill AC, Quinn C, Prichard R, McNally S. Can the synthetic C view images be used in isolation for diagnosing breast malignancy without reviewing the entire digital breast tomosynthesis data set? Irish Journal of Medical Science. 2018 9 2018 Nov;19(11):1493-1503.