New feeding tube with integrated camera designed to deliver enhanced patient safety
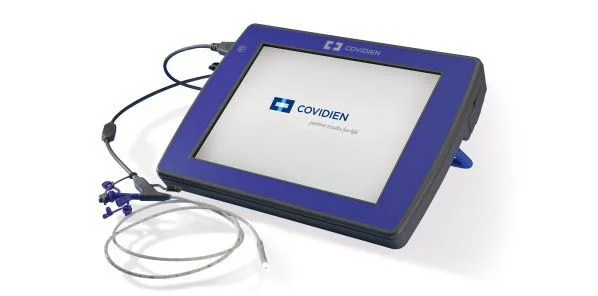
Covidien plc has announced US Food and Drug Administration 510(k) Clearance for the Kangaroo™ feeding tube with IRIS Technology. This first-of-its-kind camera-equipped disposable feeding tube is designed to enhance patient safety by providing visualisation for a procedure that is currently blinded.
The Covidien Integrated Real-time Imaging System (IRIS) technology streams a real-time video back to the Kangaroo IRIS monitor, providing visual information that can aid clinicians in identifying key areas of a patient’s anatomy. The system also enables medical professionals to save images from the live stream and make notes associated with the image.
Prior to availability of the Kangaroo feeding tube with IRIS technology, feeding tube placement was often done blindly. The risk associated with blind placement is the misplacement of the feeding tube into the patient’s airway, which can potentially cause a punctured lung or even death. The Kangaroo feeding tube with IRIS technology now gives sight where medical professionals were previously blind.
“The Kangaroo feeding tube with IRIS technology is truly unique and was built with patient safety in mind,” said Jim Clemmer, president, Medical Supplies, Covidien. “Covidien is an industry leader in nutritional delivery and enteral feeding devices and products such as the Kangaroo feeding tube with IRIS technology are designed to help improve patient outcomes and set new expectations for medical device product safety and innovation around the world.”
In addition to FDA clearance in the US, the Kangaroo feeding tube with IRIS technology is approved for use in Europe, Japan, Canada and Australia. The Kangaroo IRIS monitor is currently programmed with seven languages: English, Spanish, French, German, Italian, Portuguese and Dutch.
17 April 2014
Latest Articles
Technology, Covidien, Visualisation, tubes, feeding tube
New feeding tube with integrated camera designed to deliver enhanced patient safety Covidien plc has announced US Food and Drug Administration 510(k) Cl...