Autosomal dominant polycystic kidney disease (ADPKD) is a common inherited kidney disorder affecting millions globally, often accompanied by polycystic liver disease (PLD). PLD, characterised by liver cysts, impacts the quality of life for many ADPKD patients. Traditional methods for assessing PLD severity rely on total liver volume measurements, but they struggle to detect early-stage disease. Manual cyst identification on MRI is labour-intensive and prone to error. Current segmentation methods also face challenges. A recent study published in Radiology Advances proposes a deep learning-based approach using nn-UNet for accurate and efficient liver cyst segmentation on MRI in PLD patients. The algorithm's performance was validated against expert observers and showed promising results for routine clinical evaluations.
Evaluation of a Deep Learning Approach to Liver Cyst Segmentation for ADPKD Patients Using MRI
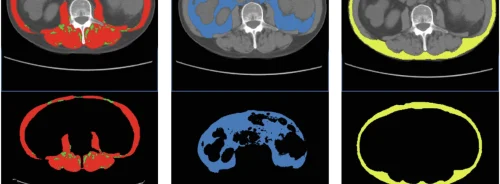
The study aimed to develop and evaluate a segmentation model for liver cysts in patients with autosomal dominant polycystic kidney disease (ADPKD) using MRI data. They analysed 197 MRI sequences from 85 patients scanned between 2017 and 2023 using routine abdominal MRI at 1.5T/3T on GE and Siemens scanners. Expert annotations were made for T1-weighted, T2-weighted, and steady-state free precession (SSFP) sequences in axial/coronal planes, reviewed by a radiologist. The nnU-Net architecture was used for the segmentation model, incorporating instance normalisation, leaky ReLU, and deep supervision. The model was trained on 40 ADPKD patients' data and evaluated on seven excluded cases, with an additional eleven cases to estimate multi-observer noise ceiling and human-level reliability. External datasets were also retrospectively analysed, and reproducibility was assessed in 17 ADPKD subjects undergoing two MRI scans within a 3-week interval. Different input modalities and training scenarios were tested, including single-channel and multi-channel inputs. Model performance was assessed using patient-level Dice (PDice) and voxel-level true positive rate (VTPR) metrics. Clinical efficiency was evaluated by measuring the time expert observers spent correcting model outputs. Inter-observer variability and human-level reliability were estimated, and test-retest reproducibility was analysed. Statistical analysis included paired Student’s t-test and Pearson correlation. Overall, the study provided a detailed analysis of liver cyst segmentation in ADPKD patients using MRI data, demonstrating the effectiveness and clinical utility of the proposed segmentation model.
Clinical Validation of Liver Cyst Segmentation Model in ADPKD
The efficiency of the deep learning framework in cyst segmentation was underscored, despite the challenge of limited labeled data. Notably, the model exhibited superior performance in predicting cyst masks for T2-weighted sequences, demonstrating its adeptness in leveraging specific MRI modalities for enhanced segmentation accuracy.
A comparative analysis of different model configurations revealed intriguing insights. Model 1, characterised by its complexity from training on various MR sequences, outperformed Model 2 in liver cyst detection. Furthermore, Model 3, which integrated liver masks as a secondary input channel, displayed a promising trend toward enhanced cyst detection. This augmentation strategy yielded notable improvements, underscoring the importance of incorporating auxiliary information for refined segmentation outcomes.
Moreover, the study meticulously evaluated the clinical efficiency of the model, showcasing substantial time reductions in expert correction when utilizing the model-assisted scenario. This underscores the practical utility of the deep learning framework in expediting diagnostic processes while maintaining high accuracy levels.
Validation on external datasets further corroborated the robustness of the model, with promising performance metrics comparable to expert radiologists. Additionally, the analysis of inter-observer variability and human-level reproducibility shed light on the inherent challenges in manual annotation, emphasising the need for automated segmentation solutions.
Furthermore, the study delved into the crucial aspect of reproducibility, assessing agreement in cyst count and fraction between initial and subsequent MRI scans. The high coefficients of correlation underscored the reliability of cyst biomarkers for tracking disease progression, especially in early stages where traditional liver volume measurements may falter.
This study represents a significant stride in the field of medical imaging, showcasing the efficacy of deep learning frameworks in liver cyst segmentation for ADPKD patients. By elucidating intricate methodologies and presenting compelling results, it contributes substantially to the ongoing pursuit of refining diagnostic methodologies in renal and hepatic pathologies.
Advancing Liver Cyst Monitoring in ADPKD: Deep Learning Solutions and Future Directions
The study underscores the importance of effective monitoring in autosomal dominant polycystic kidney disease (ADPKD), particularly regarding liver volume, which is often challenging to measure accurately, especially in early disease stages when cyst volumes are small relative to liver volume. The researchers employed a deep learning (DL) framework to streamline liver cyst annotation in ADPKD patients, reducing segmentation time significantly. They achieved a high level of accuracy in liver cyst detection, especially on axial T2-weighted sequences, while markedly reducing annotation time.
Moreover, the study observed that cyst volume measurements could track polycystic liver disease (PLD) earlier in the disease course compared to traditional height-adjusted liver volume measurements. This suggests that automated DL measurements of cyst volume could facilitate earlier detection of disease progression and enable more effective assessments of treatment efficacy.
The researchers also introduced a novel online learning medical framework to expand their training dataset iteratively, improving the model's performance with each retraining iteration. This strategy allows for the creation of ground-truth data more efficiently, facilitating the annotation of a larger number of patients with varying cyst burdens.
However, the study identified limitations in the model's performance across different MR sequences, with superior performance observed only on T2-weighted sequences. Plans to address this limitation include implementing a multi-channel model trained on multi-sequence input MR data and fine-tuning the weighting of loss functions to improve segmentation accuracy, especially at cyst boundaries.
Additionally, the study highlighted the need for more informative assessment metrics beyond Dice scores, particularly in cases with few cysts, where traditional metrics may not perform well. Future work aims to address this challenge by incorporating object-detection parameters and leveraging instance segmentation methods to enhance precision in cyst counting.
Overall, the study provides valuable insights into the potential of DL frameworks for liver cyst segmentation in ADPKD patients, offering a promising avenue for improving disease monitoring and treatment assessment. The model demonstrated high agreement with manual segmentation and high test-retest reproducibility. These findings pave the way for a more reliable and efficient quantitative biomarker to facilitate therapy development in these patients.
Source Credit: Radiology Advances
Image Credit: iStock