Microwave lung ablation (MWA) is a method for removing lung tumours with low risk and cost compared to surgery. However, it faces challenges like incomplete tumour removal due to difficulty in achieving sufficient margins. Existing methods for planning MWA based on vendor models often result in discrepancies between planned and actual outcomes. Biophysical models aiming to simulate outcomes are limited by computational complexity and lack of clinical validation. To address these issues, a new data-driven approach is proposed in European Radiology. It uses pre-procedure CT scan data, along with ablation settings, to predict the size and shape of the ablation zone post-procedure, employing a deep-neural network-based model. The aim is to improve accuracy in predicting outcomes and ensure adequate tumour removal.
Data-Driven Deep Learning for Predicting Lung Ablation Zones
A retrospective study was conducted on patients who underwent Microwave Lung Ablation (MWA) at the institution between January 2015 and January 2019, approved by the institutional review board. Data from 113 unique ablations from 72 patients were analysed. Pre-procedure and 1-month follow-up CT scans were processed, and MWA power and duration were obtained from radiology reports.
An experienced radiologist performed manual segmentation of tumour and ablation zone in pre and follow-up scans. A novel deformable image registration method was applied to register pre and follow-up scans. An applicator-centric coordinate system (ACCS) was defined for analysis across patients.
A fully convolutional neural network based on the U-Net architecture was used as the model. The network took pre-CT scan and ablation power/duration as inputs. Segmented ablation zones on follow-up scans served as ground truth for training. Nested cross-validation was employed for training and testing.
In the outer loop of cross-validation, 7-fold cross-validation was performed, and in the inner loop, the training data was further split into five train-validation splits. A total of 35 models were trained and ensembled to predict ablation zones.
Evaluation of Performance and Adherence to Patient-Specific Anatomy
The study included 72 participants with 113 MWA procedures after exclusions. Cases with poor Target Registration Error (TRE) were excluded from training/testing, resulting in 52 ablations from 40 patients. Tumor, cancer, and CT imaging details are listed in Table 1. Ablations were performed with varying power and duration, showing variability in ablation zone volumes.
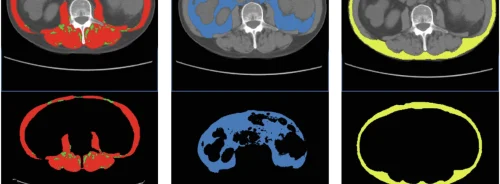
The performance of the algorithm was assessed with regards to bias and variability of volume and shape compared to the vendor model. Bland-Altman plots demonstrated lower volume bias and better agreement with the true ablation zone for the proposed prediction model compared to the vendor model. Dice scores, precision, and recall metrics also showed improvement with the proposed model, with a median Dice score of 0.62 compared to 0.56 for the vendor model.
The effect of patient-specific local anatomy on the prediction models was evaluated. The proposed model demonstrated better adherence to local anatomy, such as heat-sink effects from adjacent vessels and airways, and borders such as fissures and chest-wall boundaries, compared to the vendor model. However, there were instances where the proposed model failed to predict the ablation zone accurately, such as cases with large cavities or confounding background anatomy.
Overall, the proposed prediction model showed promising performance in predicting ablation zone size and shape, considering patient-specific anatomy, and outperformed the vendor model in several aspects.
Advancing Lung Ablation Zone Prediction: Clinical Implications of a New Data-Driven Approach
The study introduces a novel data-driven deep-learning model designed to predict lung ablation zones following Microwave Lung Ablation (MWA) procedures. This predictive model utilises pre-procedure imaging data and ablation parameters to forecast the post-procedure ablation zone appearance. The primary objective is to enhance the accuracy of predicting ablation zones compared to existing vendor models. Key findings of the study indicate several advantages of the proposed model over conventional vendor models. Firstly, the proposed model demonstrates higher Dice scores, indicative of better agreement between predicted and actual ablation zones. Additionally, it exhibits reduced variability in ablation zone volumes and diminished bias in both volume and shape compared to true ablation data.
Furthermore, the study highlights the ability of the model to better accommodate patient-specific local anatomy effects, including considerations for heat-sink effects and organ boundaries. This aspect is crucial for accurately predicting ablation zones, as it ensures that the model accounts for individual variations in anatomy that may impact the ablation process. From a clinical standpoint, the model's performance addresses the critical trade-off between precision and recall. By balancing these factors, the model aims to mitigate the risk of under-treatment, leading to local recurrence, while also avoiding overly aggressive treatment and associated complications. The study emphasises the importance of this balance in optimising patient outcomes during MWA procedures.
Despite its promising performance, the study acknowledges several limitations. These include the small size of the dataset, which may limit the model's generalizability to uncommon ablation zones. Additionally, the model assumes accurate applicator positioning, which may not always be feasible in clinical practice. However, the rapid inference time of the model renders it suitable for real-time clinical implementation. The study concludes by outlining potential clinical applications of the proposed model, such as pre-procedure planning, identification of weak points in ablation, and real-time adjustments by operators. It also suggests broader applications of the approach to other organs and treatment modalities within oncology.
Overall, the study presents a robust data-driven deep-learning approach for predicting lung ablation zones, offering significant advancements in treatment planning and prediction within the field of oncology.
Source: European Radiology
Image Credit: iStock