Breast tomosynthesis has created a lot of interest within the women’s imaging community. While the clinical benefits are becoming more apparent, the reimbursements in the U.S. are inconsistent, and it is expensive to adopt this technology. Providers want to know: Is it worth the investment? Is a positive return on investment (ROI) possible? Hologic is currently the only vendor with FDA approval for tomosynthesis; therefore, KLAS interviewed 50 Hologic tomosynthesis customers from 44 unique organisations about their experience of using the technology for at least one year.
Forty-three of 44 providers interviewed by KLAS said they would buy tomosynthesis again despite the additional time it takes and theunpredictable reimbursement. This and other findings are available in the new KLAS report Breast Tomosynthesis 2013: The Business Case.
Providers using tomosynthesis technology experience increased time and costs associated with image storage, yet providers told KLAS the benefits outweigh the costs, and they are reaping the benefits of this emerging technology.
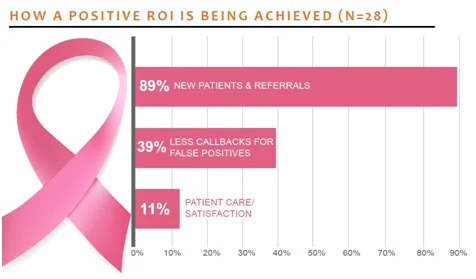
Nearly 90% of those surveyed said they are achieving a positive ROI. According to the report, providers saw "a significant reduction in false-positive callbacks, an increase in new patients, and even workflow improvements."
“This is a really exciting technology to watch evolve,” said Monique Rasband, research director and report author. “In our 2012 report on women’s imaging, we received an overwhelming response from providers to dive deeper into the clinical and financial impact of tomosynthesis. And what we have uncovered in this 2013 study will really help those who are considering tomosynthesis as a move-forward option. ”
"Hologic is pleased that KLAS has validated the reasons why so many providers are adopting 3D mammography for their patients," said Peter Soltani, Hologic's Senior Vice President and General Manager of Women's Health. "The ability to achieve a positive return on investment, while providing a leading-edge technology with demonstrated clinical advantages is a win-win situation for everyone. The recently-published Oslo Tomosynthesis Screening Trial showed that screening with Hologic's 3D mammography finds 40 percent more invasive cancers than traditional mammography alone. It is encouraging to have independent data from this report to confirm the positive business impact."
Although Hologic is the only FDA-approved vendor for tomosynthesis, many other vendors, including GE, Giotto, Philips, Planmed, and Siemens, are making progress toward tomosynthesis. The full report, Breast Tomosynthesis 2013: The Business Case is available from KLAS Research.
Latest Articles
Breast, Tomosynthesis, Hologic
Breast tomosynthesis has created a lot of interest within the women’s imaging community. While the clinical benefits are becoming more apparent, the reim...