In recent decades, significant progress has been made in biomedical and technological fields, enabling the capture of various health-related traits like molecular, genetic, metabolic, and morphological characteristics. This progress has paved the way for personalised approaches to disease management. Imaging techniques such as computed tomography, magnetic resonance imaging, and positron emission tomography play a crucial role in providing discriminating trait constellations, which are essential for personalised medicine. Advanced image post-processing and interpretation methods, including radiomics, offer a more comprehensive analysis of images and trait detection.
Despite their potential in diagnostics, therapy, prognosis, and prevention, radiomics-derived imaging biomarkers have not been widely integrated into clinical practice. This gap between research and implementation is due to various factors, including the lack of standardised workflow definitions and the absence of common terminology, which hinder comparison and reproducibility. Initiatives such as the image biomarker standardisation initiative (IBSI) and guidelines like Radiomics Quality Score (RQS) aim to address these challenges. However, a unified set of workflow definitions is still lacking.
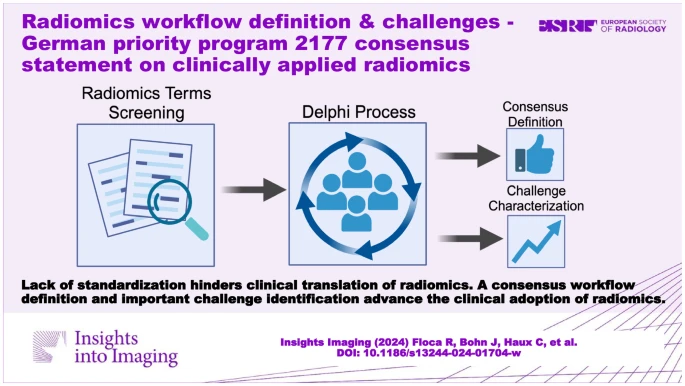
Image Credit: Insights into Imaging
To tackle this issue, the German Research Foundation's Priority Program "Radiomics" conducted a study to analyse existing workflow definitions and propose a consensus definition to enhance comparability and clarity. Through a Delphi process, the study aimed to achieve semantic analysis, propose a consensus workflow definition, and identify challenges hindering the translation of workflows into clinical practice. This collaborative effort within the scientific community is crucial for overcoming barriers and advancing the integration of radiomics into clinical routine.
A Three-Stage Approach to Refining Radiomics Workflow Definitions
This study comprised three stages to refine workflow definitions and identify challenges hindering the clinical translation of radiomics workflows.
- Definition Screening (Stage 1): This retrospective stage involved collecting workflow items and terminologies from published radiomics studies, along with reported translational challenges, to initiate a consensus-building Delphi process. A comprehensive search strategy, including PubMed and Google searches, was employed to identify relevant publications. The extracted steps were organised into a semantic hierarchy, and an initial draft for a radiomics workflow was created.
- Workflow Definition Consensus Process (Stage 2): This prospective stage utilised a structured Delphi process to achieve consensus among domain experts regarding the radiomics workflow. Five rounds of questionnaires were conducted, focusing on resolving terminology conflicts and refining a consensus definition for different aspects of the workflow.
- Challenge Characterization Process (Stage 3): The Delphi process was also used to identify and prioritise current roadblocks to the clinical translation of radiomics workflows. Two rounds focused on achieving consensus regarding the importance of different challenges and establishing a characterisation. Experts from the DFG Priority Program 2177 Radiomics, consisting of 16 projects and more than 45 interdisciplinary experts, were recruited for the Delphi process.
Overall, this study aimed to standardise radiomics terminology, refine workflow definitions, and identify key challenges to support the clinical translation of radiomics workflows, leveraging the expertise and collaboration within the DFG Priority Program 2177 Radiomics.
Consensus, Challenges, and Contributions: 51 publications screened
In this study, 51 publications were screened to extract radiomics workflow definitions, resulting in 22 publications with relevant content. A total of 95 workflow step terms were identified, with the most frequently mentioned terms listed. Validation revealed 45 conflicts, including synonyms, homonyms, hierarchy issues, and semantic ambiguities. These conflicts were addressed to create a baseline for building a consensus radiomics workflow definition, consisting of eight main steps and 28 substeps.
The workflow definition consensus process involved structured Delphi rounds, resulting in a consensus version structured into seven phases and 37 aspects. While most aspects achieved high consensus, two remained controversial regarding data format conversion and image quality assessment.
A detailed version of the workflow definition, along with a proposed formal representation as an OWL ontology, will be made publicly available. Additionally, a mapping table between the consensus definition and analysed literature terms was provided to aid translation between terms used in different publications. Notably, the radiomics standardisation guidelines ARISE, CLEAR, IBSI, and RQS were compared, with only the "Feature extraction" phase being represented in all four.
The challenge characterisation process combined expert panel proposals and literature-derived challenges to identify the ten most important challenges hindering the clinical application of radiomics workflows. Most challenges were anticipated to require solutions involving multiple domains, with medium-term solution time frames being most common.
Overall, this study contributes to standardising radiomics terminology, refining workflow definitions, and identifying key challenges to support the clinical translation of radiomics workflows.
Ten most important challenges in translating radiomics into clinical practice
The Delphi process was employed to achieve consensus on a common radiomics workflow definition. This involved multiple rounds of questionnaires, leading to a high level of agreement (89.7%) among experts regarding the proposed workflow definition. The consensus workflow definition comprised seven major phases and 37 associated aspects, providing a structured framework for conducting radiomics studies.
Additionally, the study identified and ranked the ten most important challenges in translating radiomics into clinical practice, as perceived by the participating experts. These challenges included issues such as reproducibility/generalizability, workflow integration, and the lack of guidelines for reviewers. Despite existing initiatives like IBSI and RQS, several challenges remained inadequately addressed, highlighting the need for further research and collaboration within the field.
The study acknowledged limitations, such as its geographic focus on experts from Germany and the evolving nature of machine learning-based workflows. However, it emphasised the importance of establishing a standardised definition of radiomics workflow terms and invited broader international consensus discussion to advance the field.
Overall, the study significantly contributed to the standardisation of radiomics terminology and workflow definitions, laying the groundwork for future advancements in the field and addressing the challenges hindering the clinical translation of radiomics.
Source: Insights into Imaging
Title Image Credit: iStock