Prostate-specific membrane antigen (PSMA)-targeted alpha therapy (TAT) has emerged as a promising treatment for advanced-stage metastatic castration-resistant prostate cancer (mCRPC). Since its introduction in 2014, PSMA-TAT has shown significant potential in controlling tumour growth. However, the treatment is not without challenges, particularly concerning salivary gland toxicity, which has led to dose adjustments and the exploration of combination therapies. This article explores the evolution of PSMA-TAT, focusing on its efficacy, safety, and the latest developments in treatment protocols.
Initial Successes and Challenges with AcPSMA Therapy
The initial use of 225Ac-PSMA-617 (AcPSMA) in PSMA-TAT demonstrated promising results in controlling tumour progression in mCRPC patients. A standard dosing regimen of 100 kBq of AcPSMA per kilogramme of body weight was found to be effective in achieving tumour control. However, this regimen also led to significant salivary gland toxicity, particularly xerostomia, in 10% of patients, resulting in discontinuation of therapy for some individuals. This side effect underscored the need for a more nuanced dosing strategy, especially as the treatment was extended to patients with varying degrees of tumour burden.
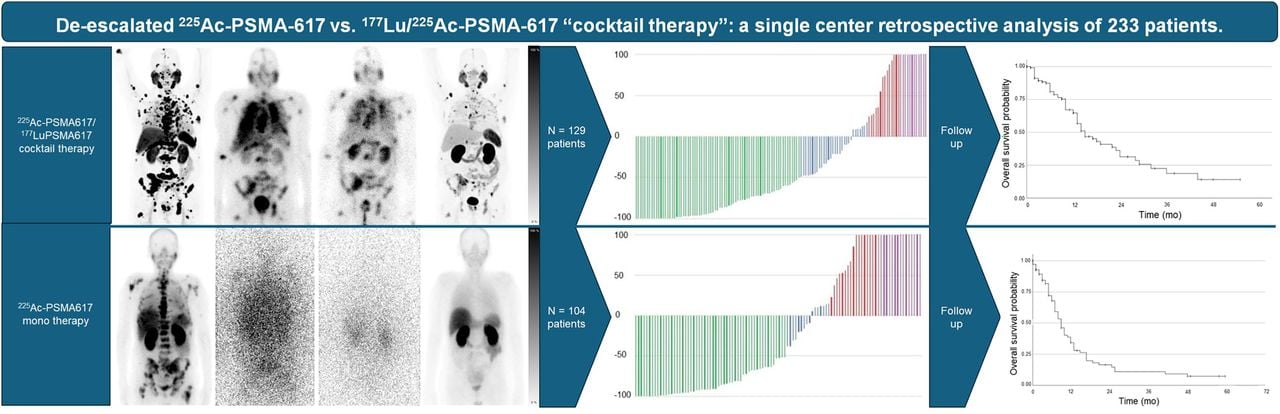
Image Source: Journal of Nuclear Medicine
Evolving Treatment Approaches: Dose De-escalation and Combination Therapies
Treatment protocols began to evolve to address salivary gland toxicity. Researchers observed a relevant tumour sink effect, especially in patients with a high tumour burden, leading to a reassessment of the maximum tolerable dose. This realisation prompted the adoption of a dynamic de-escalation approach, where initial high doses of AcPSMA were gradually reduced based on patient response and tumour burden. This approach aimed to minimise side effects while maintaining therapeutic efficacy.
In parallel, combination therapies involving 177Lu-PSMA-617 (LuPSMA) and AcPSMA were explored. Initial studies showed that combining these agents could maintain antitumor activity while reducing the incidence of xerostomia compared to AcPSMA monotherapy. Patients received varying doses of AcPSMA and LuPSMA, with some receiving additional cycles based on their responses. These combination therapies have shown promise, offering a potential pathway to optimise the balance between efficacy and tolerability.
Clinical Outcomes and Future Directions
A retrospective evaluation of patients treated with de-escalated AcPSMA and AcPSMA-LuPSMA combination therapies has provided valuable insights into the clinical outcomes of these approaches. The study included patients treated at Heidelberg University Hospital from January 2014 to July 2021, focusing on those ineligible for standard treatments and those who had exhausted other options. The results indicated that both treatment regimens achieved significant reductions in prostate-specific antigen (PSA) levels, a key marker of treatment efficacy.
Patients receiving AcPSMA monotherapy saw a median overall survival (mOS) of 9.0 months, while those receiving combination therapy with LuPSMA had an mOS of 15.0 months. Notably, the combination therapy group experienced fewer cases of severe xerostomia, suggesting that the cocktail approach might offer a safer alternative without compromising effectiveness. These findings align with other studies that have reported similar outcomes for patients treated with PSMA-targeted therapies, supporting the potential of these regimens in managing advanced prostate cancer.
The evolution of PSMA-targeted therapies, including dose deescalation and combination approaches, represents a significant advancement in the treatment of advanced mCRPC. While challenges such as salivary gland toxicity remain, the ongoing refinement of dosing strategies and the introduction of combination therapies hold promise for improving patient outcomes. Future studies with larger patient cohorts and longer follow-ups are essential to further validate these findings and optimise treatment protocols. As PSMA-TAT continues to evolve, it offers hope for better managing mCRPC, providing patients with more effective and tolerable treatment options.
Source: Journal of Nuclear Medicine
Image Credit: iStock