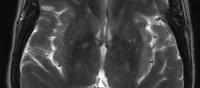
Delphinus Medical Technologies, Inc., innovator and developer of a new ultrasound platform that images the entire breast, has received 510(k) clearance from the U.S. Food and Drug Administration for the SoftVueTM whole breast ultrasound tomography system, approved for diagnostic breast imaging.
Delphinus is the first company to design and manufacture a breast ultrasound system that utilizes ring transducer technology to transmit and receive ultrasound signals.
“We believe that the opportunity now is better than ever for SoftVue’s ultrasound system to advance the state-of-the-art in breast cancer diagnostics,” commented Paul McCreadie, managing director of Arboretum Ventures and Chairman of the Board of Delphinus. “The shortcomings of existing modalities are widely known, and SoftVue’s ability to provide early and accurate diagnostic information without the risk of radiation exposure will serve to improve women’s health.”
With the breast suspended in warm water and a short exam time of one to two minutes per breast, the exam is safe, fast and comfortable. While traditional ultrasound looks at only a limited area of breast tissue, SoftVue provides a complete map of the whole breast. And unlike traditional ultrasound where the outcome of the exam is dependent on operator skill and experience, the exam is consistent and uniform, removing user variability.
Mark Morsfield, CEO of Delphinus commented “We are pleased to receive this clearance from the FDA for our innovative technology. With an experienced team dedicated to advancing breast imaging and women’s health, Delphinus is committed to assisting medical professionals better define and diagnose breast disease, while establishing a better patient experience that can be available for all women, regardless of age and without the concern of radiation or discomfort.”
Source: Arboretum Ventures
8 January 2014
Latest Articles
Screening, Imaging, Breast, Ultrasound, breast cancer
Delphinus Medical Technologies, Inc., innovator and developer of a new ultrasound platform that images the entire breast, has received 510(k) clearance fro...