Microvascular invasion is a key histopathological feature linked to recurrence and poor survival in hepatocellular carcinoma, yet it is confirmed only after surgery, limiting its value for preoperative decision-making. A retrospective multicentre analysis of 655 surgically confirmed cases explored whether three-dimensional fractal dimension derived from contrast-enhanced CT can non-invasively indicate microvascular invasion and inform prognosis. Fractal dimension, calculated from portal venous phase images, was examined alongside clinical variables to develop and validate predictive models across internal and external cohorts. The findings suggest that a quantitative imaging marker reflecting tumour morphological complexity adds measurable value to existing clinical predictors and can stratify patients by recurrence-free and overall survival risk after resection.
Three-Dimensional Fractal Metric from Routine CT
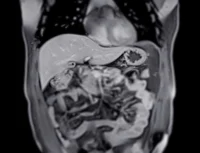
The analysis computed a three-dimensional fractal dimension using a box-counting approach on manual segmentations of the entire tumour on portal venous phase images. Image resampling to a uniform voxel size and intensity normalisation were applied to standardise data from different scanners before segmentation and feature extraction. Inter-reader reliability for fractal dimension was excellent, with an intraclass correlation coefficient of 0.98, indicating robust reproducibility of the metric. The approach captured non-integer dimensional characteristics that quantify tumour complexity across scales, aiming to reflect microvascular and morphological heterogeneity not readily appreciated by conventional semantic features.
Must Read: Predicting MVI in Hepatocellular Carcinoma with DL
Across the training, internal test and external test cohorts, fractal dimension values were consistently higher in microvascular invasion-positive tumours than in microvascular invasion-negative tumours. Corresponding areas under the curve for fractal dimension alone were 0.792 in the training set, 0.786 in the internal test set and 0.776 in the external test set, demonstrating stable discriminatory performance across sites. These results support the feasibility of a single, quantitative, scale-invariant metric derived from routine CT to indicate the likelihood of microvascular invasion before surgery.
The operational pathway does require manual three-dimensional segmentation, which dominated analysis time compared with the box-counting computation itself. While this constraint may limit immediate clinical adoption, the authors note that automated segmentation methods could mitigate the burden and improve throughput and consistency. The analysis focused on portal venous phase images, a phase reported to offer practical advantages for reproducibility in this context.
Integrating Fractal Features with Clinical Predictors
Univariable analysis identified several factors associated with microvascular invasion, including alpha-fetoprotein, alanine aminotransferase, neutrophil-to-lymphocyte ratio, albumin, tumour number, maximum tumour diameter, Edmondson-Steiner grade and fractal dimension. In multivariable logistic regression, four independent predictors remained: alpha-fetoprotein, tumour number, maximum tumour diameter and fractal dimension. These variables were integrated into a combined model that outperformed a clinical-only model across all cohorts.
In the training set, areas under the curve were 0.835 for the combined model versus 0.788 for the clinical model. In the internal test set, the combined model achieved an area under the curve of 0.795 compared with 0.752 for the clinical model. In the external test set, the combined model reached 0.826 versus 0.739 for the clinical model. DeLong tests showed significant improvements in discrimination with the addition of fractal dimension. Calibration analyses indicated good agreement between predicted and observed outcomes, and decision curve analyses demonstrated higher net benefit for the combined model across clinically relevant thresholds.
These results position fractal dimension as an objective imaging marker that complements routine preoperative variables. Unlike subjective semantic features that can suffer from interobserver variability, and unlike radiomics pipelines that require selection among large numbers of potentially unstable features, the fractal metric offers a single quantitative descriptor intended to capture tumour heterogeneity across scales. The consistent gains in external validation support its potential for broader application.
Prognostic Stratification After Curative Resection
Microvascular invasion confirmed on histology was associated with poorer recurrence-free and overall survival across cohorts. Using the combined model’s predicted risk score and an optimal cut-off, patients were stratified into high-risk and low-risk groups. In the training set, median recurrence-free survival was 28 months in the high-risk group compared with 43 months in the low-risk group. Median overall survival was 40 months in the high-risk group compared with 58 months in the low-risk group. Similar stratification patterns were observed in both the internal and external test sets.
These findings indicate that preoperative integration of fractal dimension with key clinical features can help identify patients with more aggressive disease biology who experience earlier recurrence and reduced survival after surgery. Such risk granularity may enable more targeted decisions on resection margins, perioperative therapies and postoperative surveillance intervals, within the constraints of existing institutional protocols and multidisciplinary planning.
A three-dimensional fractal dimension derived from portal venous phase contrast-enhanced CT differentiated microvascular invasion-positive from microvascular invasion-negative hepatocellular carcinoma and improved predictive performance when combined with alpha-fetoprotein, tumour number and tumour size. The metric demonstrated strong reproducibility, added diagnostic value beyond clinical factors and enabled risk stratification associated with recurrence-free and overall survival after resection. While manual segmentation currently imposes a practical bottleneck, external validation and decision-analytic gains suggest a path toward clinically useful preoperative risk estimation once workflow efficiencies are addressed. This approach offers an objective imaging marker that can complement established variables to refine patient selection, operative planning and follow-up strategies in hepatocellular carcinoma.
Source: European Radiology
Image Credit: iStock