ICU Management & Practice, Volume 22 - Issue 5, 2022
An overview of the main ultrasonographic tools that allow physiotherapists to improve their evaluation in the critical patient, described through the mnemonic PHISIO.
Introduction
Ultrasound is considered to be the fifth pillar of the physical examination to provide and improve patient care (Narula et al. 2018). In critically ill patients, the limited time to make differential diagnoses and decisions in treatment are crucial for patient survival. Point of Care of Ultrasound (POCUS) can help with these needs in the intensive care unit (ICU) by reaching an accurate diagnosis and providing adequate management, resulting in a valuable diagnostic tool (Díaz-Gómez et al. 2021; Lau and See 2022). Furthermore, POCUS applications have been gaining strength and evidence in respiratory care and physical rehabilitation. The use of this tool seems to be setting itself as a powerful ally for physical therapists treating critically ill patients.
The main ultrasonographic evaluations that will allow physiotherapists to improve their evaluation and attention in the critical patient are described below through the mnemonic “PHISIO” (Figures 1-2).
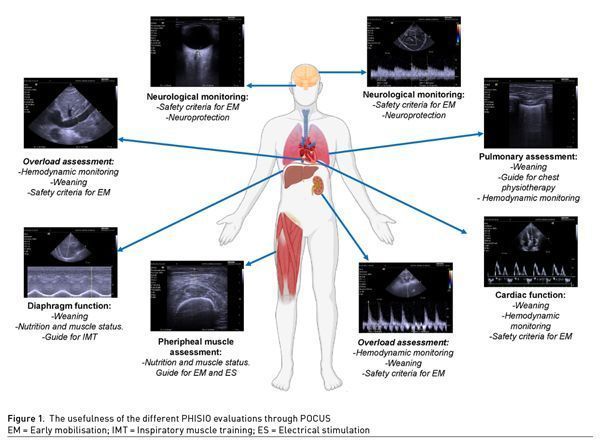
P = Pulmonary
Pulmonary ultrasound is currently feasible for the diagnosis of respiratory failure through the BLUE (Bedside Lung Ultrasound in Emergency) protocol (Leidi et al. 2020; Lichtenstein et al. 2004). However, it is useful for the physiotherapist to identify different types of ultrasound profiles to guide chest physiotherapy and in-depth respiratory monitoring (Lichtenstein et al. 2004; Le Neindre et al. 2016). According to the ultrasound profile presented by the patient, the physiotherapist can guide and apply different care strategies or treatments. For example, in the case of B-lines, non-invasive mechanical ventilation (NIV), PEP devices and active early mobilisation including verticalisation through sitting and standing can be applied. The goal would be to improve aeration and pulmonary ventilation through devices, positioning or exercise (Le Neindre et al. 2016; Hickmann et al. 2021). It is important to mention that in the case of pulmonary coalescence and subpleural consolidations, precautions should be taken into account by the rehabilitation staff. Early mobilisation (EM) or rehabilitation protocols in the ICU need to ensure the cause of respiratory failure has been stabilised to be safe. Pulmonary oedema or an infectious process can be monitored with ultrasound allowing us to observe changes over time and deciding the correct timing in the initiation of an early mobilisation programme (Le Neindre et al. 2016).
A common finding in critically ill patients can be the presence of pulmonary consolidations and will suggest pneumonia (dynamic air bronchogram) accompanied by clinical criteria, or atelectasis (static air bronchogram) (Lichtenstein et al. 2009; Sartori and Tombesi 2010). In the case of pneumonia, the effect of the antibiotic must be assessed and airway clearance techniques may be considered. On the other hand, when facing atelectasis, bronchial hygiene techniques such as those that favour peak expiratory flow (Marti et al. 2013; Amaral et al. 2020), manual or mechanical hyperinflation (Paulus et al. 2012; Assmann et al. 2016; Tucci et al. 2019), PEP devices, positioning in different decubitus, cough assistance and verticalisation (Le Neindre et al. 2016; Westerdahl et al. 2005; Volpe et al. 2018; Gates et al. 2021) can be some tools that may help the resolution of such problems. Also, pleural effusions, empyema and haemothorax can be identified faster through ultrasound compared to x-ray (Soni et al. 2015; Walsh et al. 2021). This allows early interventions such as pleural drainage accompanied by breathing exercises with PEP devices (Dos Santos et al. 2020) that improve ventilation and lung function, as well as prevent diaphragmatic dyskinesia (Le Neindre et al. 2016; Leech et al. 2015; Valenza-Demet et al. 2014). NIV should be applied with caution as it may limit lymphatic drainage and consequently pleural drainage. The use of conventional oxygen therapy devices or high-flow oxygenation therapy combined with active exercise and inspiratory muscle training (IMT) is preferable for pleural drainage due to the negative pressure generated (Walden et al. 2013).
Finally, the overall Lung Ultrasound Score (LUS) of >17 points allows us to determine the failure in the Spontaneous Breathing Trial (SBT) during the weaning process. Similarly, >6 B lines in anterolateral fields, may indicate weaning-induced pulmonary oedema (WIPO) (Santangelo et al. 2022). It is important to consider previous pulmonary pathologies and the evaluation of echocardiography for this matter.
Figure 2: Algorithm: Physiotherapy evaluation with POCUS
H = Heart
The evaluation of the haemodynamic status of the critical patient through ultrasound has become a routine practice to determine clinical stability (Santangelo et al. 2022; Kashani et al. 2022). Echocardiography may be performed before and after early mobilisation in specific conditions, as well as during the weaning of mechanical ventilation. The basic and advanced Focus Assessed Transthoracic Echo (FATE) aims at cardiopulmonary monitoring, ensuring safety before rehabilitation and for successful extubation (Kashani et al. 2022; Vieillard-Baron et al. 2019; Nagre 2019; Leidi et al. 2020).
It is important to mention that qualitative visual evaluation is one of the great competencies to be developed for the rapid detection of cardiac alterations, before carrying out specific measurements. The evaluation of the four cardiac chambers in any view allows for determining the shape, size and movement of the ventricles, atriums and septum. A right ventricle (RV) dilation can be caused by volume overload and delayed MV weaning (Denault et al. 2018). Other causes are acute pulmonary embolism (PE) which contraindicates EM in these acute phases. In this case, if dilation is enough to compress the left ventricle, D-sing appears in systole and diastole, among other signs such as non-compressible lower limb veins (Falster et al. 2022; Dabbouseh et al. 2019). This must be accompanied by clinical criteria: tachycardia, desaturation, respiratory distress, hypotension and there may be an elevation of the D-dimer. Atrial dilation may be suggestive of diastolic dysfunction and consequently failure to weaning. Pericardial evaluation is useful for the physiotherapist as moderate-severe pericardial effusion could contraindicate EM if it's accompanied by haemodynamic instability (Picano et al. 2018). In addition to the heart chambers, visualisation of large vessels such as the inferior vena cava (IVC) can be helpful to identify hypovolaemia on its collapse and dilation in fluid overload.
The systolic and diastolic functions can be assessed in a more specific and advanced way. Some examples are LV fraction shortening (LVFS), mitral annulus and tricuspid plane systolic excursion (MAPSE and TAPSE), among others (Lang et al. 2015; Hernandez-Suarez et al. 2019; Shah et al. 2019). They are complementary and useful haemodynamic measurements in shock. During the SBT an evaluation of the LV ejection fraction (LVEF) <40%, transmitral flow and tissue Doppler with their respective measurements: E/A ratio >2 and E/é >13 may indicate failed weaning (Santangelo et al. 2022; Vetrugno et al. 2020; Roche-Campo et al. 2019; Suárez et al. 2016). These measurements also allow us to obtain a more specific vision of the heart and its relationship with clinical stability during physiotherapist interventions. However, they require more training and studies supporting echocardiography in patients undergoing a rehabilitation programme in the ICU.
I = Intracranial Pressure
One of the few contraindications for early mobilisation and other physiotherapeutic interventions is elevated intracranial pressure (ICP), >20mmHg (Hernandez et al. 2021; Kumar et al. 2020; Oklowski and Shah 2017; Martínez et al. 2021). The gold standard for ICP measurement is the intraventricular catheter; however, it has some limitations (Nag et al. 2019; Hawryluk et al. 2022). A feasible alternative for neuromonitoring is measuring the diameter of the optic nerve sheath (ONSD) and the determination of the pulsatility index (PI) of the middle cerebral artery (MCA) through ultrasound (Chacko 2014; Robba et al. 2019).
A >5mm ONSD indicates ICP >20mmHg. This measurement is performed in a bilateral transorbital window without excessive pressure, avoiding damage or triggering a vagal stimulus (Cannata et al. 2022; Stead et al. 2021; Raffiz and Abdullah 2017; Aduayi et al. 2015). The evaluation of cerebral blood flow (CBF) can be performed by the sonogram and the PI. Colour Doppler and pulsed modes in the transtemporal window are required to find and display the MCA at midbrain zones (Robba et al. 2019; Lau and Arntfield 2017). Once the sonogram is obtained, its morphology and PI are evaluated to determine cerebral vascular resistance and CBF alterations. A PI 0.6-1.1 is considered normal, higher (>1.1) or lower (<0.6) values indicate elevated ICP and hyperaemia respectively (Álvarez-Fernández et al. 2009). Complementing these evaluations, the Lindergaar index can be performed with an index >3 indicating vasospasm (Hernandez et al. 2021; Robba and Taccone 2019; Robba et al. 2019).
Neuromonitoring with POCUS can be performed in any critical patient since
neuroprotection is needed in any critical scenario, especially to identify the cause of a deterioration of the acute neurological state.
S = Shock
One of the main competencies that have to be developed is the identification of shock in critically ill patients. There are clinical findings that allow us to identify a patient in shock. However, sometimes determining the cause is difficult with conventional physical examination alone (Gonzalez et al. 2020a). POCUS allows us to distinguish the cause of the state of shock in the presence of haemodynamic deterioration and consequently an intervention plan (Schmidt et al. 2012; Zieleskiewicz et al.2021; Gonzalez et al. 2020b). Some findings are tachycardia, delayed capillary filling, mottled skin, oliguria or any other clinical data of tissue hypoperfusion, considering that hypotension by itself is not synonymous with shock, but is the most common delayed manifestation (Corradi et al. 2020). There are different protocols such as RUSH and FALLS that can be applied in our daily practice (Leidi et al. 2020; Ávila-Reyes et al. 2021; Paul and Panzer 2021).
In addition, seeking free fluid in the context of trauma may indicate haemorrhage with hypovolaemic shock (Mok 2016). In patients with thoracic trauma, the identification of haemothorax, pneumothorax and cardiac tamponade are essential. The FAST-E protocol allows the identification of the main problems with abdominal, pelvic and thoracic trauma (Desai and Harris 2018). The other causes of shock such as infection, pulmonary thromboembolism and cardiac disorders will be mentioned in other sections.
Patient safety assessment for early mobilisation initiation should go beyond a checklist. POCUS provides safety in all physical therapy interventions, as well as identifying potentially lethal complications.
I = Inspiratory and Peripheral Muscles
Muscle wasting is one of the main complications that occur in critical patients. This is due to many factors, such as immobilisation, MV, and drugs (Martínez et al. 2021; Umbrello and Formenti 2016). In the case of the diaphragm, diaphragmatic dysfunction (DD) may occur and consequently increase the risk of delaying or failing in the weaning process (Goligher et al. 2019). The physiotherapist can perform diaphragmatic ultrasound (DUS), through diaphragmatic excursion (DE) and diaphragmatic thickening fraction (DTF) (Tuinman et al. 2020). During SBT, cut-off points >2 cm and 30-36% respectively are considered for successful weaning. Lower measurements are considered DD (Haaksma et al. 2022), while no movement indicates diaphragmatic paralysis. When the physiotherapist encounters these alterations and difficult weaning, he can use interventions such as IMT with linear loading devices and EM (Le Neindre et al. 2016; Haaksma et al. 2022). In this case, the DUS will become the functional-muscular monitoring in response to treatment.
Another frequent muscle complication is ICU-acquired weakness (ICU-AW), present in 32% to 80% of critically ill patients (Wang et al. 2020). This makes it susceptible to worse clinical outcomes (Goligher et al. 2019). There are different methods to assess muscle strength and status in the ICU. Muscle ultrasound (MUS) is one of them with the advantage of being applied from early stages and in non-cooperative patients. Some measurements are muscle thickness (MTh), the cross-sectional area of the rectus femoris (CSA), penation angle (PA), echogenicity employing the Heckmatt scale and greyscale analysis by histogram (Formenti et al. 2019). These measurements can be performed routinely during the critical patient's stay. A 20% and 10% decrease in MTh and CSA, respectively, indicates significant muscle loss and probable ICU-AW. In addition, the MUS allows optimising monitorisation such as nutritional contribution.
O = Overload
Fluid infusion is a common practice in the ICU around the world for early-stage resuscitation. However, it has been described that fluid overload generates complications in critical patients and even increased mortality (Perez-Nieto et al. 2021). Within fluid therapy, four phases have been described to guide the objectives (ROSE): resuscitation, optimisation, stabilisation and evacuation (Malbrain et al. 2022). While the physiotherapist does not direct fluid resuscitation, difficulties can be encountered in the progression of patient functionality due to poor fluid management and fluid overload (Perez-Neito et al. 2021).
Irrational fluid use may increase the severity of critical patient respiratory involvement, even in those with MV (Ogbu et al. 2015). Among the main respiratory complications are pulmonary oedema, pleural effusion, alteration of pulmonary compliance, reduction of the PaO2/FiO2 ratio, increase MV days and difficult weaning. Moreover, the presence of pulmonary B lines suggests pulmonary oedema; however, fluid overload is not the only cause. A targeted evaluation should be done to rule out other causes such as heart failure with LV alterations, inflammatory process (e.g., ARDS) or WIPO. Visualisation of ICV is a good start to differentiate between these possible causes: a dilation or diameter >2 cm indicates volume overload (Argaiz et al. 2021). The complementary evaluation of venous congestion by Venous Excess Ultrasound Score (VEXUS) can be useful. VEXUS protocol evaluates the flow of the hepatic, portal vein and intra-renal veins to identify such congestion (Rola et al. 2021; Galindo et al. 2021).
In the context of physiotherapy and respiratory progression, the presence of pulmonary B lines dilated IVC with a collapse index <50% or altered VEXUS suggests fluid overload (Argaiz et al. 2021; Galindo et al. 2021). Volume clearance should be considered to see if there would be an improvement in ventilatory parameters, the use of diuretics or haemodialysis in case of renal injury may be alternative in the treatment (Beaubien-Souligny et al. 2020).
Although human beings are formed mostly by water, this does not mean that it can’t harm the critically ill. Everything can be harmful with an inadequate dose; fluid therapy is no exception.
Conflict of Interest
None.
References:
Aduayi OS, Asaleye CM, Adetiloye VA et al. (2015) Optic nerve sonography:A noninvasive means of detecting raised intracranial pressure in a resource-limited setting. Journal of Neurosciences in Rural Practice. 6(4):563-567.
Álvarez-Fernández JA, Martín-Velasco MM, Igeño-Cano JC, Pérez-Quintero R (2010) Utilidad del Doppler transcranealen la resucitación de la paradacardíaca. MedicinaIntensiva. 34(8):550-558.
Argaiz ER, Koratala A, Reisinger N (2021) Comprehensive Assessment of Fluid Status by Point-of-Care Ultrasonography. Kidne 360. 2(8):1326-1338.
Ávila-Reyes D, Acevedo-Cardona AO, Gómez-González JF et al. (2021) Point-of-care ultrasound in cardiorespiratory arrest (POCUS-CA): narrative review article. The Ultrasound Journal. 13(1):46.
Assmann CB, Vieira PJ, Kutchak F et al. (2016) Lung hyperinflation by mechanical ventilation versus isolated tracheal aspiration in the bronchial hygiene of patients undergoing mechanical ventilation. RevistaBrasileira de TerapiaIntensiva. 28(1):27-32.
Barjaktarevic I, Kenny JS, Berlin D, Cannesson M (2021) The Evolution of Ultrasound in Critical Care:From Procedural Guidance to Hemodynamic Monitor. Journal of Ultrasound in Medicine. 40(2):401-405.
Beaubien-Souligny W, Rola P, Haycock K et al. (2020) Quantifying systemic congestion with Point-Of-Care ultrasound:development of the venous excess ultrasound grading system. The Ultrasound Journal. 12(1):16.
Blanco P (2020) Rationale for using the velocity-time integral and the minute distance for assessing the stroke volume and cardiac output in point-of-care settings. The Ultrasound Journal, 12(1):21.
Cannata G, Pezzato S, Esposito S, Moscatelli A (2022) Optic Nerve Sheath Diameter Ultrasound:A Non-Invasive Approach to Evaluate Increased Intracranial Pressure in Critically Ill Pediatric Patients. Diagnostics. 12(3):767.
Chacko J (2014) Optic nerve sheath diameter: An ultrasonographic window to view raised intracranial pressure? Indian journal of critical care medicine. 18(11):707-708.
Claure-Del Granado R, Mehta RL (2016) Fluid overload in the ICU:evaluation and management. BMC Nephrol. 109.
Corradi F, Via G, Tavazzi G (2020) What's new in ultrasound-based assessment of organ perfusion in the critically ill:expanding the bedside clinical monitoring window for hypoperfusion in shock. Intensive Care Medicine. 46(4):775-779.
Dabbouseh NM, Patel JJ, Bergl PA (2019) Role of echocardiography in managing acute pulmonary embolism. Heart (British Cardiac Society). 105(23):1785-1792.
Denault AY, Langevin S, Lessard MR et al. (2018) Transthoracic echocardiographic evaluation of the heart and great vessels. Évaluationéchocardiographiquetransthoracique du cœur et des grands vaisseaux. Canadian Journal of Anaesthesia. 65(4):449-472.
Desai N, Harris T (2018) Extended focused assessment with sonography in trauma. BJA Education. 18(2):57-62.
Díaz-Gómez JL, Mayo PH, Koenig SJ (2021) Point-of-Care Ultrasonography. The New England Journal of Medicine. 385(17):1593-1602.
Dos Santos E, da Silva JS, de Assis Filho M et al. (2020) Adding positive airway pressure to mobilisation and respiratory techniques hastens pleural drainage:arandomised trial. Journal of Physiotherapy. 66(1):19-26.
Formenti P, Umbrello M, Coppola S et al. (2019) Clinical review: peripheral muscular ultrasound in the ICU. Ann. Intensive Care. 9, 57.
García X, Mateu L, Maynar J et al. (2011) Estimating cardiac output. Utility in the clinical practice. Available invasive and non-invasive monitoring. MedicinaIntensiva. 35(9):552-561.
Goligher EC, Brochard LJ, Reid WD et al. (2019) Diaphragmatic myotrauma:a mediator of prolonged ventilation and poor patient outcomes in acute respiratory failure. The Lancet. Respiratory Medicine. 7(1):90-98.
Gonzalez JM, Ortega J, Crenshaw N, de Tantillo L (2020a) Rapid Ultrasound for Shock and Hypotension: A Clinical Update for the Advanced Practice Provider: Part 1. Advanced Emergency Nursing Journal. 42(4):270-283.
Gonzalez JM, Ortega J, Crenshaw N, de Tantillo, L (2020b) Rapid Ultrasound for Shock and Hypotension: A Clinical Update for the Advanced Practice Provider: Part 2. Advanced Emergency Nursing Journal. 42(4):284-292.
Haaksma ME, Smit JM, Boussuges A et al. (2022) Expert consensus On Diaphragm UltraSonography in the critically ill (EXODUS): a Delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Critical Care. 26(1):99.
Hawryluk G, Citerio G, Hutchinson P et al. (2022) Intracranial pressure:current perspectives on physiology and monitoring. Intensive Care Medicine. 48(10):1471-1481.
Hernandez S, Kittelty K, Hodgson CL (2021) Rehabilitating the neurological patient in the ICU:what is important?. Current Opinion in Critical Care. 27(2):120-130.
Hernandez-Suarez DF, Lopez-Menendez F, Roche-Lima A, Lopez-Candales A (2019) Assessment of Mitral Annular Plane Systolic Excursion in Patients With Left Ventricular Diastolic Dysfunction. Cardiology Research. 10(2):83-88.
Hickmann CE, Montecinos-Munoz NR, Castanares-Zapatero D et al. (2021) Acute Effects of Sitting Out of Bed and Exercise on Lung Aeration and Oxygenation in Critically Ill Subjects. Respiratory Care. 66(2):253-262.
Kumar MA, Romero FG, Dharaneeswaran K (2020) Early mobilization in neurocritical care patients. CurrOpin Crit Care. 26(2):147-154.
Lin JJ, Chen AE, Lin EE et al. (2020) Point-of-care ultrasound of optic nerve sheath diameter to detect intracranial pressure in neurocritically ill children - A narrative review. Biomedical Journal. 43(3):231-239.
Lau VI, Arntfield RT (2017) Point-of-care transcranial Doppler by intensivists. Critical Ultrasound Journal. 9(1):21.
Lau YH, See KC (2022) Point-of-care ultrasound for critically-ill patients:A mini-review of key diagnostic features and protocols. World Journal of Critical Care Medicine 11(2):70-84.
Lang RM, Badano LP, Mor-Avi V et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 28(1):1-39.e14.
Leech M, Bissett B, Kot M, Ntoumenopoulos G (2015) Physiotherapist-initiated lung ultrasound to improve intensive care management of a deteriorating patient and prevent intubation:a case report. Physiotherapy Theory and Practice. 31(5):372-376.
Leidi A, Rouyer F, Marti C et al. (2020) Point of care ultrasonography from the emergency department to the internal medicine ward: current trends and perspectives. Internal and Emergency Medicine. 15(3):395-408.
Le Neindre A, Mongodi S, Philippart F, Bouhemad B (2016) Thoracic ultrasound:Potential new tool for physiotherapists in respiratory management. A narrative review. Journal of Critical Care. 31(1):101-109.
Lichtenstein DA (2014) Lung ultrasound in the critically ill. Annals of Intensive Care. 4(1):1.
Lichtenstein D, Goldstein I, Mourgeon E et al. (2004) Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 100(1):9-15.
Lichtenstein D, Mezière G, Seitz J (2009) The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest. 135(6):1421-1425.
Martínez CM, Jones BRA, Gómez GA et al. (2021) Movilizacióntempranaen la Unidad de CuidadosIntensivos. Med Crit. 35(2):89-95.
Malbrain MLNG, Martin G, Ostermann M (2022) Everything you need to know about deresuscitation. Intensive Care Med.
Mao JY, Zhang HM, Liu DW, Wang XT (2022) Visual Rounds Based on Multiorgan Point-of-Care Ultrasound in the ICU. Frontiers in Medicine. 9, 869958.
Martí JD, Li Bassi G, Rigol M et al. (2013) Effects of manual rib cage compressions on expiratory flow and mucus clearance during mechanical ventilation. Critical Care Medicine. 41(3):850-856.
Mok KL (2016) Make it SIMPLE: enhanced shock management by focused cardiac ultrasound. Journal of Intensive Care. 4, 51.
Nag DS, Sahu S, Swain A, Kant S (2019) Intracranial pressure monitoring: Gold standard and recent innovations. World Journal of Clinical Cases. 7(13):1535-1553.
Nagre AS (2019) Focus-assessed transthoracic echocardiography:Implications in perioperative and intensive care. Annals of Cardiac Anaesthesia. 22(3):302-308.
Narula J, Chandrashekhar Y, Braunwald E (2018) Time to Add a Fifth Pillar to Bedside Physical Examination:Inspection, Palpation, Percussion, Auscultation, and Insonation. JAMA Cardiology. 3(4):346-350.
Olkowski BF, Shah SO (2017) Early Mobilization in the Neuro-ICU:How Far Can We Go? Neurocrit Care. 27(1):141-150.
Ogbu OC, Murphy DJ, Martin GS (2015) How to avoid fluid overload. Current Opinion in Critical Care, 21(4):315-321.
Paul JA, Panzer O (2021) Point-of-care Ultrasound in Cardiac Arrest. Anesthesiology, 135(3):508-519.
Paulus F, Binnekade JM, Vroom MB, Schultz MJ (2012) Benefits and risks of manual hyperinflation in intubated and mechanically ventilated intensive care unit patients: a systematic review. Critical Care. 16(4):R145.
Perez Nieto OR, Wong A, Lopez Fermin J et al. (2021) Aiming for zero fluid accumulation:First, do no harm. Anaesthesiology Intensive Therapy. 53(2):162-178.
Picano E, Scali MC, Ciampi Q, Lichtenstein D (2018) Lung Ultrasound for the Cardiologist. JACC. Cardiovascular Imaging. 11(11):1692-1705.
Price S, Platz E, Cullen L et al. (2017) Expert consensus document:Echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nature Reviews. Cardiology. 14(7):427-440.
Raffiz M, Abdullah JM (2017) Optic nerve sheath diameter measurement:a means of detecting raised ICP in adult traumatic and non-traumatic neurosurgical patients. The American Journal of Emergency Medicine. 35(1):150-153.
Robba C, Taccone FS (2019) How I use Transcranial Doppler. Crit Care. 23, 420.
Robba, C, Goffi, A, Geeraerts T et al. (2019) Brain ultrasonography:methodology, basic and advanced principles and clinical applications. A narrative review. Intensive Care Med. 45, 913-927.
Roche-Campo F, Bedet A, Vivier E et al. (2018) Cardiac function during weaning failure: the role of diastolic dysfunction. Annals of Intensive Care. 8(1):2.
Rola P, Miralles-Aguiar F, Argaiz E. et al. (2021) Clinical applications of the venous excess ultrasound (VExUS) score: conceptual review and case series. Ultrasound J. 13, 32.
Santangelo E, Mongodi S, Bouhemad B, Mojoli F (2022) The weaning from mechanical ventilation:a comprehensive ultrasound approach. Current Opinion in Critical Care. 28(3):322-330.
Sartori S, Tombesi P (2010) Emerging roles for transthoracic ultrasonography in pulmonary diseases. World Journal of Radiology. 2(6):203-214.
Schmidt GA, Koenig S, Mayo PH (2012) Shock. Chest. 142(4):1042-1048.
Sivakorn C, Schultz MJ, Dondorp AM (2021) How to monitor cardiovascular function in critical illness in resource-limited settings. Current Opinion in Critical Care. 27(3):274-281.
Soni NJ, Franco R, Velez MI et al. (2015) Ultrasound in the diagnosis and management of pleural effusions. Journal of Hospital Medicine, 10(12):811-816.
Stead GA, Cresswell FV, Jjunju S et al. (2021) The role of optic nerve sheath diameter ultrasound in brain infection. eNeurological Sci. 23, 100330.
Suárez JC, López P, Mancebo J, Zapata L (2016) Diastolic dysfunction in the critically ill patient. MedicinaIntensiva. 40(8):499-510.
Tucci MR, Nakamura MA, Carvalho NC, Volpe MS (2019) Manual Hyperinflation: Is It Effective?. Respiratory Care. 64(7):870-873.
Tuinman PR, Jonkman AH, Dres, M et al. (2020) Respiratory muscle ultrasonography: methodology, basic and advanced principles and clinical applications in ICU and ED patients—a narrative review. Intensive Care Med. 46:594-605.
Umbrello M, Formenti P (2016) Ultrasonographic Assessment of Diaphragm Function in Critically Ill Subjects. Respiratory Care. 61(4):542-555.
Valenza-Demet G, Valenza MC, Cabrera-Martos I et al. (2014) The effects of a physiotherapy programme on patients with a pleural effusion:a randomized controlled trial. Clinical rehabilitation. 28(11):1087-1095.
Vetrugno L, Guadagnin GM, Brussa A et al. (2020) Mechanical ventilation weaning issues can be counted on the fingers of just one hand:part 1. The Ultrasound Journal. 12(1):9.
Vieillard-Baron A, Millington S.J, Sanfilippo F et al. (2019) A decade of progress in critical care echocardiography:a narrative review. Intensive Care Med. 45, 770-788.
Volpe MS, Naves JM, Ribeiro GG et al. (2018) Airway Clearance With an Optimized Mechanical Insufflation-Exsufflation Maneuver. Respiratory Care, 63(10):1214-1222.
Walden AP, Jones QC, Matsa R, Wise MP (2013) Pleural effusions on the intensive care unit; hidden morbidity with therapeutic potential. Respirology. 18(2):246-254.
Walsh MH, Zhang KX, Cox EJ et al. (2021) Comparing accuracy of bedside ultrasound examination with physical examination for detection of pleural effusion. The Ultrasound Journal. 13(1):40.
Wang W, Xu C, Ma X et al. (2020) Intensive Care Unit-Acquired Weakness: A Review of Recent Progress With a Look Toward the Future. Front Med. 7:559789.
Westerdahl E, Lindmark B, Eriksson T et al. (2005) Deep-breathing exercises reduce atelectasis and improve pulmonary function after coronary artery bypass surgery. Chest. 128(5):3482-3488.
Winkler MH, Touw HR, van de Ven PM et al. (2018) Diagnostic Accuracy of Chest Radiograph, and When Concomitantly Studied Lung Ultrasound, in Critically Ill Patients With Respiratory Symptoms: A Systematic Review and Meta-Analysis. Critical Care Medicine. 46(7):e707-e714.
Zieleskiewicz L, Lopez A, Hraiech S. et al. (2021) Bedside POCUS during ward emergencies is associated with improved diagnosis and outcome:an observational, prospective, controlled study. Crit Care. 25, 34.












