ICU Management & Practice, Volume 21 - Issue 3, 2021
In addition, tracking changes in the peripheral perfusion index (PI), a variable derived from the pulse oximetry waveform, may help to detect tissue hypo-perfusion, as well as guide fluid management. In the future, pulse oximetry may also be useful to quantify respiratory efforts and to detect changes in blood pressure.
Remote and Self-Monitoring of SpO2 From Home
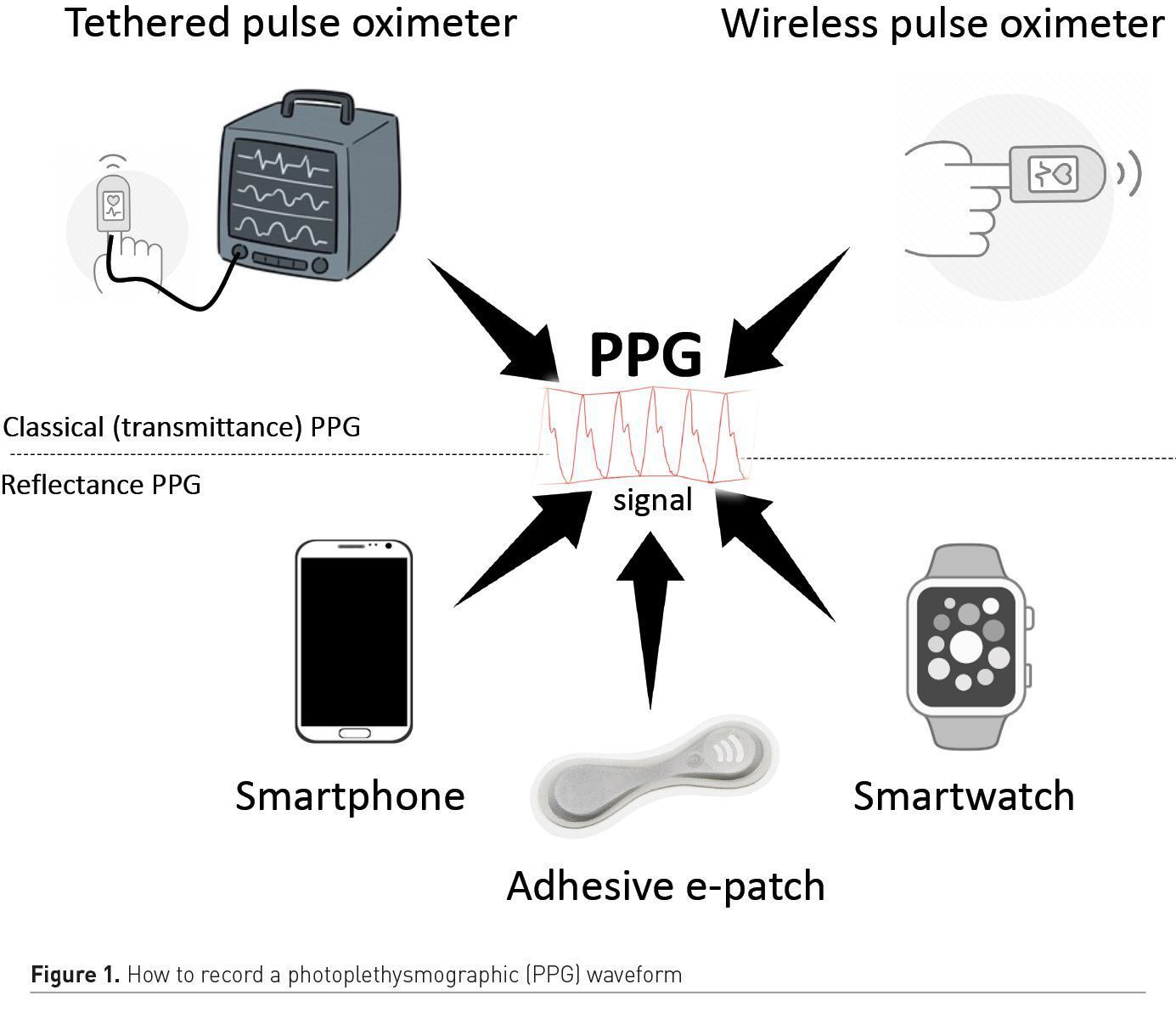
Over the last decade, pulse oximeters have been miniaturised and have become wireless. They also became affordable, and several medical grade products can now be ordered online for less than €100. As a result, pulse oximeters are more often part of our home medicine cabinet and the COVID-19 pandemic definitely boosted their adoption. Home monitoring enables the early detection of patients requiring oxygen and hospitalisation, or the surveillance of patients after early discharge from the hospital (Shah et al. 2020). For patients unable to monitor themselves, several remote monitoring programmes have been created. They usually combine the use of a finger wireless sensor by the patient and a supervision by a dedicated and remote command centre receiving SpO2 and pulse rate measurements via the patient’s smartphone. In case of abnormalities detected by the pulse oximeter, a dedicated staff is available to advise the patient on a 24/7 basis. In addition to classical finger sensors using transmittance photoplethysmography, reflective sensors part of adhesive patches, wrist devices or watches are now available (Figure 1).
In theory, these tools have potential to expand the use of pulse oximetry. However, their accuracy remains to be established by well-designed clinical studies and most have not been approved for medical use.
Continuous Monitoring on Hospital Wards
The recent surge of hospitalised COVID-19 patients created both ICU bed and healthcare worker shortages, raising concerns regarding patient safety. Therefore, the adoption of remote monitoring systems, specifically designed for hospital wards, dramatically increased. These systems enable the continuous monitoring of vital signs, including SpO2, in a large number of ward patients at the same time, display the information on a central station, and, in case of clinical deterioration, immediately alert nurses directly on their pager or cellphone (Michard et al. 2019). During epidemics, remote monitoring also has the advantage of decreasing the number of physical interactions and thereby the risk of virus transmission.
In a large study done in the medico-surgical wards of 360 US hospitals (Lyons et al. 2020) before the pandemic, over 20% of rapid response interventions were triggered by a decrease in SpO2. On the wards, vital sign spot-checks are usually done every 4-8h and another study showed that because of the intermittent nature of SpO2 measurements, nurses may miss up to 90% of hypoxaemic events (Sun et al. 2015). In this context, continuous pulse oximetry systems, able to inform nurses directly on their pager in case of clinical deterioration, have been shown to be useful to decrease the number of rescue interventions and ICU transfers. Continuous monitoring of SpO2 and other vital signs was already on the rise before the pandemic, and is today, more than ever, considered as a major opportunity to improve patient safety (Vincent et al. 2018).
Automated oxygen administration
In patients hospitalised for respiratory failure who require supplemental oxygen, precise manual oxygen titration is difficult to achieve and is time-consuming. Automated oxygen titration devices have been developed to avoid periods of hypoxaemia and hyperoxaemia (L’her et al. 2017).
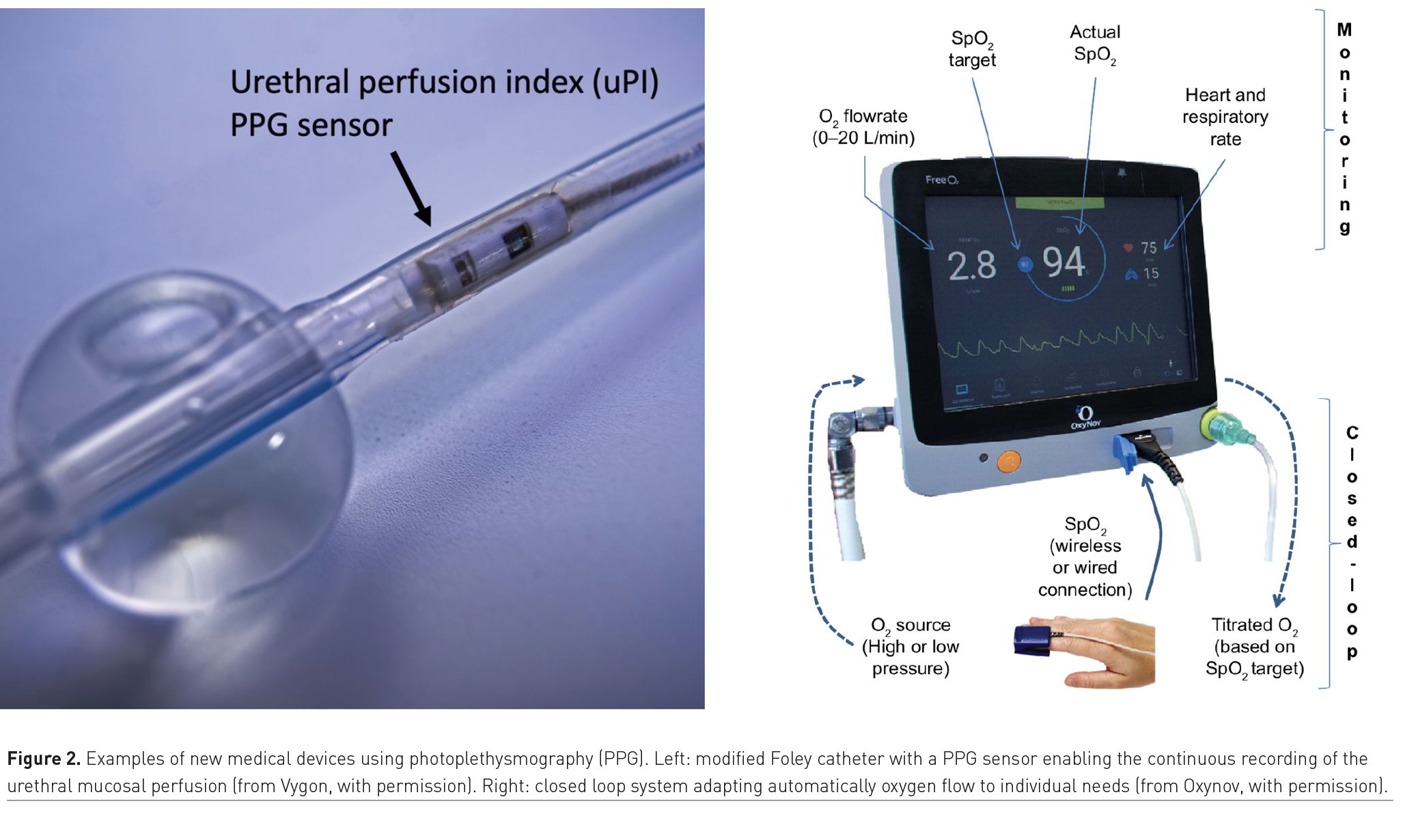
These systems are based on a closed-loop circuit that enables oxygen flow titration (the output variable) according to the patient’s real-time SpO2 (the input value). It intends to reverse the paradigm from a constant oxygen flow with a variable SpO2 value, to a constant target SpO2 set by the physician with continuous oxygen flow variations (Figure 2). The implementation of these closed-loop devices has been associated with a greater percentage of time spent within the SpO2 target range, as well as shorter duration of oxygen administration and hospital length-of-stay (Denault et al. 2019).
Assessment of tissue perfusion
The PI is the pulsatile component of the photoplethysmographic waveform. In healthy volunteers, it represents between 0.5 and 5% of the non-pulsatile signal. The PI is calculated and displayed by many pulse oximeters and, until recently, was mainly used as a quality indicator for pulse oximetry. Monitoring PI has been proposed to assess the peripheral tissue perfusion. It is often low (<0.5%) in patients with shock or receiving vasopressors, has been shown to correlate with lactate and venous oxygen saturation and to be an independent risk factor for 30-day mortality (He et al. 2015).
Pulse oximeters are typically positioned on fingers or ears so that the PI provides information about the local perfusion. Sometimes, pulse oximeters are used on toes to monitor limb perfusion after vascular surgery or during veno-arterial extra-corporeal membrane oxygenation. A new Foley catheter, incorporating a photoplethysmographic sensor, was recently developed to continuously monitor the urethral PI (Dépret et al. 2020). By detecting early changes in pelvic perfusion, it may help to optimise haemodynamics during high-risk abdominal surgery and in shock states (Figure 2).
 Other methods have been proposed to assess peripheral tissue perfusion or muscle oxygenation. They include the clinical evaluation of the capillary refill time and the assessment of tissue oxygenation by non-infrared spectroscopic (NIRS) sensors. The evaluation of the capillary refill time is intermittent and NIRS sensors are expensive. Whether they have any advantages to guide haemodynamic therapy over the mere and continuous monitoring of PI with a pulse oximeter remains to be demonstrated.
Other methods have been proposed to assess peripheral tissue perfusion or muscle oxygenation. They include the clinical evaluation of the capillary refill time and the assessment of tissue oxygenation by non-infrared spectroscopic (NIRS) sensors. The evaluation of the capillary refill time is intermittent and NIRS sensors are expensive. Whether they have any advantages to guide haemodynamic therapy over the mere and continuous monitoring of PI with a pulse oximeter remains to be demonstrated.
Fluid Management
During general anaesthesia
In anaesthetised and mechanically ventilated patients, the respiratory changes in left ventricular stroke volume induce proportional changes in arterial pulse pressure (PPV) and in PI, known as the pleth variability index (PVI). PVI has been shown to be a good predictor of fluid responsiveness in the operating room, but not in the ICU where motion artifacts are frequent, and the pulse oximetry signal is sometimes far to be optimal (as often evidenced by a low PI).
Because intra-operative PPV-guided fluid therapy has been shown to decrease postoperative morbidity, a recent study (Fischer et al. 2020a) investigated the value of PVI to guide fluid therapy during surgery. This randomised controlled trial assessed the postoperative outcome impact of maintaining PVI <13% during surgery. The study did not show any outcome benefits but the percentage of time within target (PVI < 13%) was low (< 40% of the surgery time) and did not differ between groups. Further studies are therefore needed to clarify the possible impact of PVI-guided fluid strategies on postoperative outcome. However, it is important to bear in mind that all non-invasive attempts made so far to rationalise fluid therapy during surgery, either with PVI or cardiac output monitoring systems, failed to impact postoperative outcome (Fischer et al. 2020b). These findings may simply reflect the fact that because postoperative complications are uncommon in low-risk surgical patients, there is not much room for improvement. In other words, if goal directed fluid therapy with PPV or invasive pulse contour methods has been shown to be useful to improve postoperative outcome in high-risk surgical patients (who usually have a radial catheter in place), it is unlikely that goal directed fluid therapy with non-invasive methods may be cost-effective in low-risk patients.
In patients with acute respiratory failure
In patients with acute respiratory failure, individualised fluid therapy is desirable to balance the risks of fluid overload (increase in pulmonary oedema) with the risks of fluid restriction (decrease in cardiac output and oxygen delivery to the tissues). Predicting preload responsiveness is a way to identify patients who may benefit from fluid administration and, maybe more importantly, to prevent unjustified fluid boluses in preload non-responders. Predicting preload responsiveness was recently recommended by WHO, the Surviving Sepsis Campaign guidelines and the NIH for the fluid management of COVID-19 patients.
In spontaneously breathing patients and during protective mechanical ventilation, it is well established that both PPV and PVI have a poor predictive value, which is mainly related to a low sensitivity. In contrast, the assessment of changes in cardiac output during a passive leg raising (PLR) manoeuvre has been shown to accurately predict preload responsiveness. This approach requires the use of cardiac output monitoring systems which are not always readily available or used, particularly in spontaneously breathing patients. Interestingly, the two main determinants of the pulse oximetry-derived PI are vascular tone and cardiac output. Assuming no significant changes in vascular tone, one may therefore assume that changes in PI may reflect changes in cardiac output. A recent study done in critically ill patients showed that a relative increase in PI >9% during a PLR manoeuvre predicts an increase in cardiac output >10% with a sensitivity of 91% and a specificity of 79% (Beurton et al. 2019). In summary, when cardiac output is not monitored, PLR-induced changes in PI may help to predict fluid responsiveness.
What’s Next?
Quantifying respiratory efforts
The physiologic response to hypoxaemia is an increase in respiratory frequency and tidal volume. Work of breathing increases, resulting in large changes in pleural pressure which may be responsible for self-inflicted acute lung injury. This phenomenon has been advocated to explain, at least in part, the rapid deterioration of lung function in severe COVID-19 patients. A recent study (Tonelli et al. 2020) suggests that patients with acute respiratory failure in whom respiratory efforts do not quickly decrease after initiating non-invasive ventilation, ultimately require tracheal intubation. In research studies, respiratory efforts are quantified by monitoring the respiratory swings in oesophageal pressure. But in clinical practice, oesophageal probes are not used. In spontaneously breathing patients, PVI depends mainly on the magnitude of changes in pleural pressure and could therefore be used to approximate respiratory efforts (Michard and Shelley 2020). Thus, when initiating oxygen therapy or non-invasive ventilation, monitoring changes in PVI may help to assess the impact on respiratory efforts and has potential to prevent intubation delays. Clinical studies are currently ongoing to confirm these hypotheses.
Tracking changes in blood pressure
Pulse oximeters can be used to calculate the pulse wave transit time (PWTT), which is the time difference between cardiac contraction and the peripheral pulse arrival. The PWTT depends on blood flow and the mechanical properties of the arterial bed. Both a decrease in cardiac output and in vascular tone (the two determinants of blood pressure) induce an increase in PWTT. Thus, tracking changes in PWTT has been proposed to predict changes in blood pressure. For instance, continuous monitoring of PWTT has been shown to be useful to detect beat-to-beat changes in systolic arterial pressure during anaesthesia induction (Kim et al. 2013). It could therefore be used to predict hypotension and automatically trigger oscillometric brachial cuff measurements. In ward patients wearing wireless electrodes (to detect cardiac contraction) and a finger pulse oximeter, tracking changes in PWTT has been used to unmask hypotensive events that, otherwise, would have been missed by nurses who were spot-checking blood pressure only every 4h (Turan et al. 2019).
More recently, a machine learning algorithm has been developed to predict blood pressure from photoplethysmographic waveforms (Ghamri et al. 2020). A large number of pulse oximetry waveforms and corresponding invasive blood pressure numbers were used to “teach” the algorithm how to recognise specific patterns or signatures of blood pressure changes (learning phase). During the validation phase, the algorithm demonstrated an excellent performance to detect changes in arterial pressure during anaesthesia induction.
Conclusion
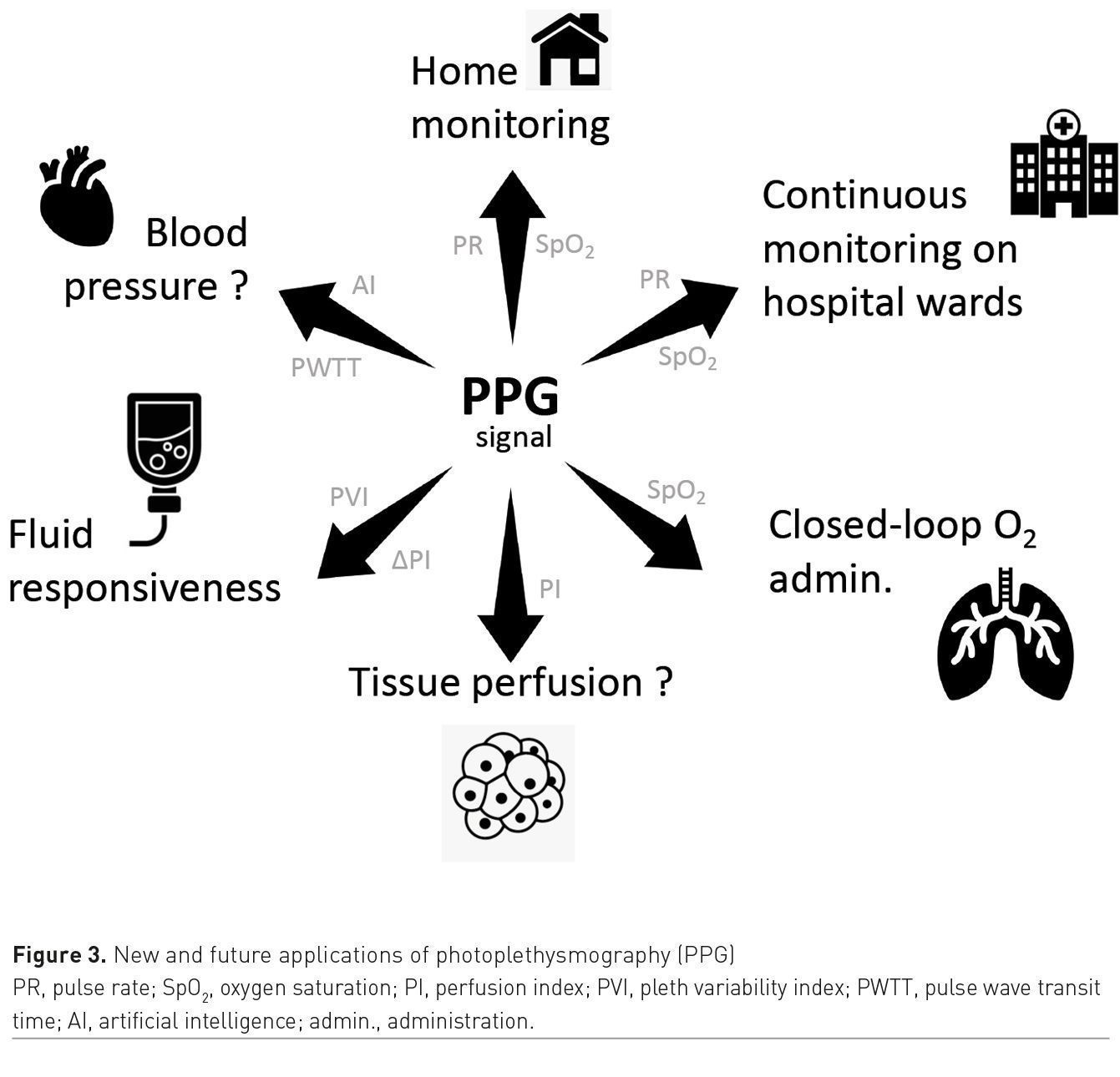
The surge of patients with COVID-19 has been a catalyst for the adoption of SpO2 monitoring from home, remote and continuous monitoring of vital signs on hospital wards and closed-loop administration of oxygen (Figure 3). In patients with acute respiratory failure, tracking changes in PI during a PLR manoeuvre may help to identify fluid non-responders and hence prevent unjustified fluid administration. In spontaneously breathing patients, a decrease in PVI may reflect a decrease in respiratory efforts. Therefore, monitoring PVI may help to assess the efficacy of oxygen therapy, CPAP or non-invasive ventilation. Changes in PWTT predict changes in blood pressure and could be used to trigger upper-arm cuff measurements (automatic smart triggering). Machine learning systems have potential to extract more information from pulse oximetry waveforms, and such waveforms can now be recorded by smartwatches or adhesive patches. These innovations should further push the envelope of photoplethysmography and create new opportunities for physiologic monitoring beyond the operating room and the ICU.
Conflict of Interest
FM is the founder and managing director of MiCo (michardconsulting.com), a Swiss consulting and research firm. MiCo does not sell any medical device and FM does not receive royalties from any medical
device company.
References:
Beurton A, Teboul JL, Gavelli F et al. (2019) The effects of passive leg raising may be detected by the plethysmographic oxygen saturation signal in critically ill patients. Crit Care, 23:19.
Denault MH, Péloquin F, Lajoie AC, Lacasse Y (2019) Automatic versus manual oxygen titration in patients requiring supplemental oxygen in the hospital: A systematic review and meta-analysis. Respiration, 98:178-188.
Dépret F, Leone M, Duclos G et al. (2020) Monitoring tissue perfusion: a pilot clinical feasibility study of a urethral photoplethysmography-derived perfusion device in high-risk patients. J Clin Monit Comput, 34:961-9.
Fischer MO, Lemoine S, Tavernier B et al. (2020) Individualized fluid management using the pleth variability index: a randomized clinical trial. Anesthesiology, 133:31-40.
Fischer MO, Fiant AL, Debroczi S et al. (2020) Perioperative non-invasive hemodynamic optimization using photoplethysmography: a randomized controlled trial and
meta-analysis. Anaesth Crit Care Pain Med., 39:421-8.
Ghamri Y, Proença M, Hofmann G et al. (2020) Automated pulse oximeter waveform analysis to track changes in blood pressure during anesthesia induction: a proof-of-concept study. Anesth Analg., 130:1222-33.
He H, Long Y, Liu D et al. (2015) Clinical classification of tissue perfusion based on the central venous oxygen saturation and the peripheral perfusion index. Crit Care,19:330.
Kim SH, Song JG, Park JH et al. (2013) Beat-to-beat tracking of systolic blood pressure using noninvasive pulse transit time during anesthesia induction in hypertensive patients. Anesth Analg., 116:94-100.
L’Her E, Dias P, Gouillou M et al. (2017) Automatic versus manual oxygen administration in the emergency department. Eur Respir J., 50: pii: 1602552.
Lyons PG, Edelson DP, Carey KA et al. (2019) Characteristics of rapid response calls in the United States: An analysis of the first 402,023 adult cases from the Get with the guidelines resuscitation-Medical emergency team registry. Crit Care MeD., 47:1283-89.
Michard F, Bellomo R, Taenzer A (2019) The rise of ward monitoring: opportunities and challenges for critical care specialists. Intensive Care Med., 45:671-73.
Michard F, Shelley K (2020) Should we monitor pulsus paradoxus via pulse oximetry in COVID-19 patients with acute respiratory failure? Am J Respir Crit Care Med,, 202:770-71.
Shah S, Majmudar K, Stein A et al. (2020) Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med., 27:681-92.
Sun Z, Sessler DI, Dalton JE et al. (2015) Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg., 121:709-15.
Tonelli R, Fantini R, Tabbi L et al. (2020) Inspiratory effort assessment by esophageal manometry early predicts noninvasive ventilation outcome in de novo respiratory failure: a pilot study. Am J Respir Crit Care Med.,202:558-67.
Turan A, Chang C, Cohen B et al. (2019) Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology., 130:550-9.
Vincent JL, Einav S, Pearse R et al. (2018) Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol., 35:325-33.