ICU Management & Practice, Volume 22 - Issue 1, 2022
Introduction
While we have improved intra-operative outcomes, our patients continue to suffer harm in the postoperative period. The 30-days after non-cardiac surgery is a major cause of death in the United States and the world over (Bartels et al. 2013). Around 70% of these deaths occur before patients go home, during initial hospitalisation in the postoperative period and while they recover in our best hospital systems. Importantly, approximately one-half of all these adverse events occur in the relatively under-monitored hospital ward environment (De Vries et al. 2008; Pearse et al. 2012; Andersen et al. 2016). Most important contributions to post-operative patient mortality come from sepsis, major bleeding, and myocardial injury (Spence et al. 2019). Of these, intra and postoperative hypotension are strongly associated with myocardial injury, renal injury, and death (Walsh et al. 2013; Mascha et al. 2015; Salmasi et al. 2017; Sessler and Khanna 2018; Liem et al. 2020; Gregory et al. 2021; Khanna et al. 2021). The relationship of post-operative hypotension (POH) with myocardial injury appears more robust than intraoperative hypotension (IOH) (Sessler and Khanna 2018; Sessler et al. 2018; Liem et al. 2020; Khanna et al. 2021). POH is also strongly associated with several serious and costly adverse outcomes such as death, increased hospital length of stay, prolonged critical care needs, delirium, and kidney injury (Smischney et al. 2020; Khanna et al. 2021).
During surgery we monitor frequently (typically at least once every five minutes) for hypotension and blood pressure fluctuations according to standards set by the American Association of Anesthesiologists (ASA). The standard non-invasive technique for BP monitoring is the upper arm cuff auscultatory method developed by Korotkoff (Paskalev et al. 2005). Arterial cannulation is the usual gold standard for beat-to-beat and invasive blood pressure monitoring, that detects at least two times as much hypotension as intermittent cuff monitoring in the intra-operative environment (Naylor et al. 2020). Substantial new data has emerged that proves accuracy and validation of non-invasive and portable alternatives for arterial lines (Martina et al. 2012; Ameloot et al. 2013; Gratz et al. 2017; Tanioku et al. 2020; Kwon et al. 2021). Consequently, the scope of accurate blood pressure monitoring, and prevention of harm related to haemodynamic changes is now extending beyond the traditional confines of the operating room, the post-anaesthesia care unit, and the intensive care unit.
Haemodynamic monitoring for patients during immediate postoperative recovery in the PACU is frequent as well and may include for some, a more enhanced monitoring phase in the ICU. However, this monitoring standard drops off rapidly as patients are transitioned to hospital ward care where at best vital signs are checked every once in 4-8 hours (Khanna et al. 2019; Turan et al. 2019). This leaves the patient unmonitored for most of the hospital stay after surgery (Sessler and Saugel 2019; Khanna et al. 2021). A wrong yet tempting assumption here, is that with increased time after surgery and delivery of anaesthesia there is a reduced risk of influencing the patient’s cardiovascular or respiratory homeostasis and that most patients are on track to normal physiology and an uneventful clinical recovery.
Adverse cardiorespiratory events occur commonly on hospital wards, importantly most do not occur suddenly, instead are preceded by hours of progressively more abnormal vital signs (Andersen et al. 2016) Because vital signs are measured intermittently, postoperative blood pressure and heart rate perturbations are often sustained for long periods without recognition (Turan et al. 2019) However, published studies have been small, restricted to selected populations, and involve blinded clinicians to supplemental monitoring. We as yet miss an adequately powered randomised trial to test the influence of postoperative hypotension monitoring on patient centric outcomes (Andersen et al. 2016; Turan et al. 2019; Weenk et al. 2019, 2020; Liem et al. 2020).
Building a Continuous Monitoring System on Hospital Wards
Some important questions need answered as we build an effective continuous blood pressure monitoring system for the hospital ward. There are many proposed definitions of hypotension or what is constituted as clinically relevant low blood pressure in the postoperative patient. While several different thresholds of blood pressure and components have been investigated, a question that remains yet to be answered for hospital ward patients is, if there is an absolute blood pressure level, or a relative blood pressure compared to a (mostly unknown) baseline blood pressure that is more critical to outcomes. Most commonly, during the intra-operative period an absolute level of 65mmHg of mean arterial pressure (MAP) is a widely accepted level whereas another definition is 30% below baseline MAP, both of which appear to have a similar risk (Salmasi et al. 2017). This threshold for the hospital ward patients seems somewhat higher at a MAP of 75 mmHg (Liem et al. 2020; Khanna et al. 2021). Do we view hypotension as a singular insult or a cumulative burden with a dose dependent effect on organ damage? While most of the published thresholds have been established with frequent intra-operative blood pressure data, it is difficult to replicate the same experiments, with a normal ward monitoring regimen as intermittent spot checks are far too interspersed to translate into a cumulative effect (Khanna et al. 2021).
Perioperative hypotension is associated with increased healthcare resource utilisation (Stapelfeldt et al. 2021). The degree of monitoring a patient receives, reflects the perceived level of risk during the postoperative setting and is subject to a cost-benefit evaluation. One can expect the level of risk for adverse haemodynamic events to be inversely proportional to the time elapsed from surgery as the patient returns to a baseline physiology without the need for haemodynamic monitoring. As the factors in the cost-benefit equation differs so should the result of the decision of how patients are monitored. Less risk aversity would imply a higher benefit in the equation. In addition, public trust in healthcare systems is important and avoidance of adverse events are critical to build that trust. Furthermore, the development of new and accurate, well validated technologies for patient monitoring would relieve the nursing staff of manual BP measurements (and other vital signs checks). Therefore, it is very much possible to introduce more portable continuous automated monitoring systems on hospital wards, along with increased acceptance and adoption of technology and gradually decrease staffing needs. Hospital systems administrators need to understand that while there is an initial cost to implement continuous monitoring, this is soon offset with a very minimal and largely attainable decrease in adverse events (Khanna et al. 2021). Knowing how common myocardial injury is in the post-operative period and its very strong association with hypotension, a breakeven point on investment in continuous portable haemodynamic monitoring would be easily attainable in a short period of time post implementation. From that point onward, improved patient safety, less organ system injury, decreased hospital length of stay, and most importantly improved provider and patient or patient family satisfaction would drive further adoption. The most important piece in this futuristic look is the acceptance of new technology by bedside providers which will necessitate better alarm management, understanding artefacts, improving protocol-based management for haemodynamic instability, and developing platforms that act as central monitoring stations with an effective ‘efferent arm’. The role of artificial intelligence will be more important as we build a preventive and predictive arm to hypotension on the general hospital care floor as well.
Real World Postoperative Hypotension Monitoring Data
At the Atrium Health Wake Forest Baptist Medical Center part of the Wake Forest University School of Medicine, we have used continuous portable vital signs monitoring systems on our general hospital wards for about the last five years. Here, we record blood pressure and heart rate at 15-second intervals using a wireless non-invasive monitor in adults recovering from noncardiac surgery. For monitoring, we use a portable wrist mounted system that is cleared by the United States Food and Drug Administration. The system includes a 3- or 5-lead electrocardiogram and an oscillometric blood pressure monitor which is used to calibrate the continuous non-invasive blood pressure monitor at least once daily. Continuous blood pressure is estimated from pulse arrival time, specifically the time that elapses between R wave being detected and arrival of the resulting pulse at the SpO2 finger sensor.
Based on several alarm simulation studies, we currently have the monitor generate nursing alerts for haemodynamic events defined by heart rate >150 beats/minute or <39 beats/minute, systolic blood pressures >200 mmHg or <80 mmHg and mean arterial pressures (MAP) <58 mmHg. These settings have allowed us to capture significant vital signs disturbances while limiting alarm fatigue. Nurses are encouraged to intervene when clinically indicated and have an escalating system of networked alarms. The monitors are calibrated at least daily and connected to the hospital’s wireless network. Vital signs abnormalities exceeding established thresholds generate alerts that are distributed to a central station and to the nurses’ hospital-supplied phones. Alarms that are not addressed by the primary nurses within a few minutes are escalated to other floor nurses, and thereafter to the unit manager.
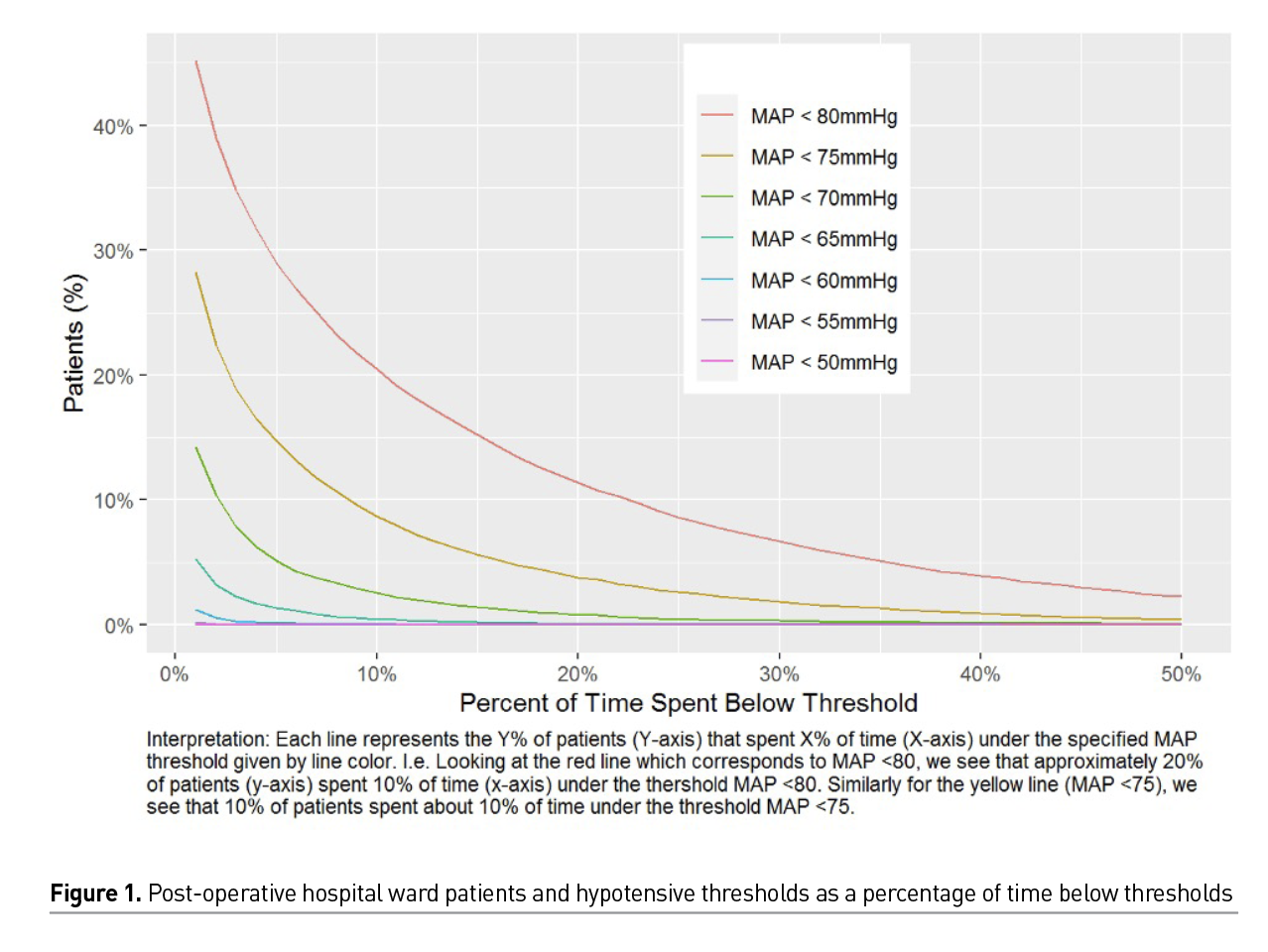
Our recently analysed data sample contains 82715 monitoring sessions across 31587 patient visits among 28108 total patients (Unpublished data - Khanna and colleagues). While our hospital wide continuous and ‘closed loop’ monitoring systems achieved better results than previously published small datasets with blinded monitoring (Turan et al. 2020), we still see significant hypotension that is picked up by continuous monitoring that would have gone unrecognised with intermittent monitoring. Figure 1 shows percentage of patients by time spent hypotensive across varying defining thresholds; here roughly 20% of our patient population spent at least 10% of their time hypotensive defined conservatively by MAP < 80mmHg. Slightly fewer than 10% of patients spent at least 10% of their time with MAP < 75mmHg, and fewer than 5% of patients spent at least 10% of time spent with MAP < 70mmHg.
Assessing Figure 2, we see the relationship of continuous minutes of monitoring time spent under blood pressure thresholds for proportions of monitored patients. We had approximately 34% of patients spend at least one minute with MAP ≤ 70mmHg, and another approximately 20% of patients who spent at least five continuous minutes with MAP ≤ 70mmHg. The ‘Intermittent Detection Incidence’ (dashed line) closely follows the line representing the incidence rate of patients with hypotension defined by sustained periods of time > 30 minutes for each threshold. This suggests that intermittent patient assessments every four hours would capture about the same amount of hypotensive episodes as continuous monitoring when a hypotensive episode is defined as spending at least 30 minutes below a given threshold.
Existing Technologies
Pulse Wave Velocity (PWV) measures time delay of a pulse wave from its origin in the heart, defined by the ECG signal, and its detection at the finger through a pulse oximetry reading (Rastegar et al. 2020; Senturk et al. 2020). Derivation of blood pressure from the time delay between ECG and plethysmograph is more complex than a simple correlation. Algorithms that consider signal quality, artefacts and perfusion are in place to convert a measurement of time delay to one of pressure. These algorithms have been trained on large ICU datasets such as the Medical Information Mart for Intensive Care (MIMIC-II) (Senturk et al. 2020). Studies comparing the technique with a cuff-based technique and arterial line have shown good calibration (Watanabe et al. 2017; Hill et al. 2021) while validation against an invasive arterial line appears to be lacking (Hill et al. 2021). In theory, several modalities can be used to collect proximal and distal waveforms, such as speckle plethysmogram, impedance plethysmogram or mechanical pulse wave (Le et al. 2020; Pielmus et al. 2021). Pulse wave velocity or pulse arrival time systems have been implemented clinically with good results and with minimal alarm fatigue at some healthcare systems in the United States including ours (Weller et al. 2018). We report some of the processed data from several thousand patients at our healthcare system in the previous section.
Pulse wave decomposition is another established method that relies on a morphological analysis of the plethysmograph wave form. This may be in effect an advancement on pulse contour analysis of the arterial wave form established in critical care (Baruch et al. 2014; Pielmus et al. 2021). The method itself is based on breaking down the plethysmograph waveform into its different components and analysing them both individually and as a composite measure based on relative size and time delay at the sensor level. After calibration, this method delivers a reliable blood pressure reading validated against an intra-operative radial arterial cannulation (Gratz et al. 2017; Kwon et al. 2021).
Volume clamp method relies on a finger cuff that is continuously inflated to keep the artery at constant size as measured by the absorption of light. The pressure delivered is used as a basis to estimate pressure at the level of the brachial artery. This system has excellent validation data and as well as data that has shown an increase in detection and a decrease in overall hypotension (more corrective measures as detection increased) when used in the operating room compared to standard intermittent cuff based measures (Martina et al. 2012; Maheshwari et al. 2018; Tanioku et al. 2020).
Optical pulse wave analysis technology is analogous to pulse wave decomposition but uses a photo plethysmography wave form as its input. A bracelet housing the optical emitter and sensor is worn on the wrist. Systems that are available have shown good calibration in recent studies (Nachman et al. 2020; Vybornova et al. 2021).
Newer Technologies
Artery applanation is based on an automation of the clinical practice of palpating a pulse at a convenient location such as the radial artery at the wrist. A highly sensitive pressure transducer converting the minute mechanical energy to an electric signal complete with waveform is achieved.
It is however sensitive to sensor placement and movement artefacts (Földi et al. 2018). Current iterations are bulky and not in routine use, but there is work being done to miniaturise the technology.
Continuous wave (CW) doppler ultrasound patches that measure flow velocity over the carotid artery are under development and show promising results (Kenny et al. 2021). By attaching this device over the carotid artery and keeping it in place with an adhesive, a CW doppler signal can be collected continuously and haemodynamic data can be extracted.
Electrical conductance of the thorax is associated with the proportion of fluid it contains. As pulsatile blood flow is the dominating source of fluctuation of fluid volume there is an association between blood flow in the thorax and the measured conductance. Further research is warranted for these to be used as long term portable monitoring (Nguyen and Squara 2017).
Electrical cardiometry derives cardiac output and thereby blood pressure from measuring electrical impedance changes from orientation of red blood cells in pulsatile flowing blood. This is achieved through a series of electrical sensors on the thorax, neck, and thigh (Sanders et al. 2020).
A Look to the Future
The availability of wireless continuous blood pressure monitoring devices is increasing. Several systems are in place using different technological approaches. A higher level of haemodynamic monitoring of patients after surgery extending beyond the PACU and ICU seems inevitable. A culture change that will necessitate increased accountability and responsibility for correction of haemodynamic changes using higher intensity monitoring is necessary and has already begun. The future of monitoring will take it beyond the hospital and home with the patient. Several interesting questions need answered. How do we build effective closed loop continuous monitoring systems on hospital wards with minimal alarm fatigue, best provider, and patient acceptance as well as a maximal decrease in adverse events? How do we take the patient from continuous ward monitoring to no monitoring whatsoever on hospital discharge? Will the transition from continuous monitoring in the hospital to home monitoring mean ‘less-frequent’ continuous monitoring as a ‘weaning’ mechanism? Will postoperative monitoring at home have a central monitoring system and be linked to billable hospital services for providers? Will it influence us to discharge patients earlier from the hospital after surgery or will we paradoxically keep patients in the hospital longer because we detect more changes in vital signs with a higher level of monitoring? As monitors move from direct measurements to derived values, often with the help of advanced algorithms, there arises a need to ‘monitor the monitors’. Alarm fatigue is a real threat as well as the need to detect technical issues and disconnections. By integrating a huge number of datapoints, the possibility of automating early warning scores seems natural and necessary. Beyond digitising and automatisation of existing early warning scores, there is the possibility to use continuous streaming physiological vital signs data patterns to make real-time predictions for clinical outcomes and events. A set of haemodynamic parameters can potentially dynamically be analysed not as a selection of individual values but in relation to each other. Here a set of measurements that each in their own is within normal range can still potentially signal an impending deterioration.
As is the case with many emerging technological advances in the field of anaesthesia and critical care, this field is also driven by the advent of artificial intelligence (AI) or machine learning. It is the possibility of taking large amounts of data and developing algorithms correlating the current state input signal to an estimation of haemodynamic compromise in future. Artificial intelligence is also needed to determine if an out of bounds measurement is due to a clinically important haemodynamic change or the result of a technical issue. Given the enormous amounts of data our patients generate in the peri- and postoperative setting, AI is taking on a greater role in helping clinicians be aware of significant clinical developments at the same time shielding them from sifting through large amounts of noisy data.
Predicting new technology is difficult; however we can be certain that existing ward monitoring technology will be refined, and hardware will be further miniaturised and ultimately there will be universal adoption and growth to improve patient safety outcomes. With growing interest in self-monitoring, it is also likely that perhaps the consumer and asks from our hospitalised patients will lead the way forward in the next five years. A well designed, appropriately powered large randomised trial with the right outcomes will most certainly be that landmark paper that will place continuous blood pressure monitoring on floor patients as part of guidelines documents.
Conflict of Interest
Dr Olsen has no conflict of interest to report. Dr Khanna consults for Edwards Lifesciences, Philips North America, GE Healthcare, Hill-Rom, Potrero Medical, Retia Medical and Caretaker Medical. His institution has current and recent grant funding from Edwards Lifesciences, Caretaker Medical, Potrero Medical and Retia Medical for ongoing investigations on portable monitoring. Dr Khanna is on the executive advisory board for Medtronic. He receives support from the Wake Forest CTSI via NIH/NCATS KL2 for a trial of continuous portable haemodynamic and saturation monitoring on hospital wards. Dr Khanna is a founding member of the BrainX group that conducts education, research, and collaboration on AI techniques in healthcare and has a commercial arm at www.BrainXAI.com. 

References:
Ameloot K et al. (2013) Nexfin Noninvasive Continuous Hemodynamic Monitoring: Validation against Continuous Pulse Contour and Intermittent Transpulmonary Thermodilution Derived Cardiac Output in Critically Ill Patients. The Scientific World Journal. Edited by L.M. Gillman et al.p. 519080.
Andersen LW et al. (2016) The prevalence and significance of abnormal vital signs prior to in-hospital cardiac arrest. Resuscitation. 98:112–117.
BartelsK et al. (2013) Perioperative organ injury. Anesthesiology. 119(6):1474–1489.
Baruch MC et al. (2014) Validation of the pulse decomposition analysis algorithm using central arterial blood pressure. BioMedical Engineering OnLine. 13(1):96.
De VriesENet al. (2008) The incidence and nature of in-hospital adverse events: a systematic review. BMJ Quality & Safety. 17(3):216–223.
FöldiS et al. (2018) A novel non-invasive blood pressure waveform measuring system compared to Millar applanation tonometry. Journal of Clinical Monitoring and Computing, 32(4):717–727.
GratzI et al. (2017) Continuous Non-invasive finger cuff CareTaker® comparable to invasive intra-arterial pressure in patients undergoing major intra-abdominal surgery. BMC Anesthesiology.17(1):48.
GregoryA et al. (2021) Intraoperative hypotension is associated with adverse clinical outcomes after noncardiac surgery. Anesthesia & Analgesia. 132(6):1654–1665.
HillBL et al. (2021) Imputation of the continuous arterial line blood pressure waveform from non-invasive measurements using deep learning. Scientific reports. 11(1):1–12.
KennyJÉSet al. (2021) A novel, hands-free ultrasound patch for continuous monitoring of quantitative Doppler in the carotid artery. Scientific Reports. 11(1):7780.
KhannaAK et al. (2021) Postoperative Hypotension and Adverse Clinical Outcomes in Patients Without Intraoperative Hypotension, After Noncardiac Surgery. Anesthesia & Analgesia. pp. 10–1213.
KhannaAK, HoppeP, SaugelB (2019) Automated continuous noninvasive ward monitoring: future directions and challenges. Critical Care, 23(1):194.
KwonY et al. (2021) Tracking of the beat-to-beat blood pressure changes by the Caretaker physiological monitor against invasive central aortic measurement. Blood Pressure Monitoring [Preprint].
Le T et al. (2020) Continuous Non-Invasive Blood Pressure Monitoring: A Methodological Review on Measurement Techniques. IEEE Access. 8:212478–212498.
LiemVG et al. (2020) Postoperative hypotension after noncardiac surgery and the association with myocardial injury.. Anesthesiology. 133(3):510–522.
MaheshwariK et al. (2018) A randomized trial of continuous noninvasive blood pressure monitoring during noncardiac surgery. Anesthesia and analgesia. 127(2):424.
MartinaJRet al. (2012) Noninvasive continuous arterial blood pressure monitoring with Nexfin®. Anesthesiology. 116.
MaschaEJet al. (2015) Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology. 123(1):79–91.
NachmanD et al. (2020) Comparing blood pressure measurements between a photoplethysmography-based and a standard cuff-based manometry device. Scientific Reports. 10(1):16116.
NaylorAJ et al. (2020) Arterial catheters for early detection and treatment of hypotension during major noncardiac surgery: a randomized trial. Anesthesia & Analgesia. 131(5):1540–1550.
NguyenLS, SquaraP (2017) Non-invasive monitoring of cardiac output in critical care medicine. Frontiers in medicine. 4:200.
PaskalevD, Kircheva A,KrivoshievS. (2005) A centenary of auscultatory blood pressure measurement: a tribute to Nikolai Korotkoff.Kidney & blood pressure research. 28(4):259–263.
PearseRM et al. (2012) European Surgical Outcomes Study (EuSOS) group for the Trials groups of the European Society of Intensive Care Medicine and the European Society of Anaesthesiology. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 380(9847):1059–65.
PielmusAG et al. (2021) Surrogate based continuous noninvasive blood pressure measurement. Biomedical Engineering/Biomedizinische Technik. 66(3):231–245.
RastegarS, GholamHosseiniH, LoweA (2020) Non-invasive continuous blood pressure monitoring systems: current and proposed technology issues and challenges. Physical and Engineering Sciences in Medicine. 43(1):11–28.
SalmasiVet al. (2017) Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 126(1):47–65.
Sanders M, Servaas S, Slagt C (2020) Accuracy and precision of non-invasive cardiac output monitoring by electrical cardiometry: a systematic review and meta-analysis. Journal of Clinical Monitoring and Computing. 34(3):433–460.
SenturkU, PolatK, YucedagI (2020) A non-invasive continuous cuffless blood pressure estimation using dynamic Recurrent Neural Networks. Applied Acoustics. 170:107534.
SesslerDIet al. (2018) Period-dependent Associations between Hypotension during and for Four Days after Noncardiac Surgery and a Composite of Myocardial Infarction and Death: A Substudy of the POISE-2 Trial.Anesthesiology. 128(2):317–327.
SesslerDI, KhannaAK (2018) Perioperative myocardial injury and the contribution of hypotension. Intensive care medicine. 44(6):811–822.
SesslerDI, SaugelB(2019) Beyond failure to rescue: the time has come for continuous ward monitoring. British Journal of Anaesthesia. 122(3):304–306.
SmischneyNJ et al. (2020) Postoperative hypotension in patients discharged to the intensive care unit after non-cardiac surgery is associated with adverse clinical outcomes. Critical Care. 24(1):1–12.
SpenceJ et al. (2019) Association between complications and death within 30 days after noncardiac surgery. Cmaj. 191(30):E830–E837.
StapelfeldtWH et al. (2021) Association of perioperative hypotension with subsequent greater healthcare resource utilization. Journal of Clinical Anesthesia. 75:110516.
TaniokuT et al. (2020) Validation of noninvasive continuous arterial pressure measurement by ClearSightSystemTM during induction of anesthesia for cardiovascular surgery. BMC anesthesiology. 20(1):176.
TuranAet al. (2019) Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology. 130(4):550–559.
TuranA et al. (2020) Mild acute kidney injury after noncardiac surgery is associated with long-term renal dysfunction: a retrospective cohort study. Anesthesiology. 132(5):1053–1061.
VybornovaAet al. (2021) Blood pressure from the optical Aktiia Bracelet: A 1-month validation study using an extended ISO81060-2 protocol adapted for a cuffless wrist device. Blood Pressure Monitoring. 30:000–000.
WalshM et al. (2013) Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension.Anesthesiology. 119(3):507–515.
Watanabe N et al. (2017) Development and Validation of a Novel Cuff-Less Blood Pressure Monitoring Device. JACC: Basic to Translational Science. 2(6):631–642.
WeenkM et al. (2019) Wireless and continuous monitoring of vital signs in patients at the general ward. Resuscitation. 136:47–53.
WeenkMet al. (2020) Continuous monitoring of vital signs in the general ward using wearable devices: randomized controlled trial. Journal of medical Internet research. 22(6):e15471.
WellerRS, FoardKL, HarwoodTN (2018) Evaluation of a wireless, portable, wearable multi-parameter vital signs monitor in hospitalized neurological and neurosurgical patients. Journal of Clinical Monitoring and Computing. 32(5):945–951.








