ICU Management & Practice, Volume 17 - Issue 3, 2017
Presents the results from a randomised controlled trial which aimed to determine if fibrinogen concentrate infusion reduces intraoperative blood loss in cardiac surgery patients.
Background
Excessive bleeding is a common complication in cardiac surgery, and may result in the need for red blood cell (RBC) transfusion. Intraoperative bleeding during cardiac surgery is often treated with coagulation factor concentrates (CFCs). As yet, however, their efficacy has not been conclusively determined.
Since 1980 our knowledge of damage from transfusion has increased. Risks include infection, effects on the immune system, transfusion-related acute lung injury and risks due to the age of transfused blood (Isbister et al. 2011). Healthcare systems need to also be aware of the associated costs. As doctors it is our challenge and duty to reduce preventable damage from blood transfusion.
Patient blood management
The evidence-based multidisciplinary approach to optimising care of patients who may need transfusion is known as patient blood management (PBM). We know there is considerable variation in perioperative blood transfusion rate. For example, an analysis of 102,470 patients who underwent coronary artery bypass graft surgery at 792 hospitals in the United States found that rates of blood transfusion ranged from 7.8% to 92.8% for red blood cells (Bennett-Guerrero et al. 2010). PBM can also reduce the need for blood transfusions. In cardiac surgery, a study of Jehovah’s Witnesses, who refuse blood products, found no difference in morbidity and mortality if patients are evaluated with a multidisciplinary approach to blood management (Moraca et al. 2011).
At Isala Clinics, we have researched patient blood management, including tailor-made transfusion protocols (Bilecen et al. 2014), and the role of point-of-care testing and fibrinogen concentrate (Bilecen et al. 2013). We implemented a specific transfusion protocol for cardiac surgery, and conducted an intervention study to evaluate its effects on transfusion and clinical events (Bilecen et al. 2014). The protocol included giving component therapy and fibrinogen at the end of the schedule. If we measured fibrinogen less than 1g/L we added 2g of extra fibrinogen. If it was more than 1g/L based on the Clauss measurement, we did not give fibrinogen. The cardiac surgery-specific transfusion protocol resulted in fewer patients transfused with RBCs and fresh frozen plasma (FFP) and a lower incidence of myocardial infarction.
Fibrinogen concentrate therapy
We conducted a cohort study to evaluate the effect of fibrinogen concentrate therapy on postoperative blood loss and transfusion and occurrence of clinical events in complex cardiac surgery; 264/1075 patients received fibrinogen concentrate during surgery (Bilecen et al. 2013). There was no reduction in postoperative blood loss and transfusion (intensive care unit [ICU] blood loss: OR 1.02 (0.91-1.14) and ICU transfusion: OR 1.14 (0.83-1.56) and no increase in risk for adverse clinical events. However, the haemostatic effect may have been attenuated by the low doses and relatively late administration of fibrinogen concentrate therapy.
Randomised controlled trial
Therefore we initiated a prospective single-centre, randomised, placebo-controlled, doubleblind clinical trial to find out if fibrinogen concentrate infusion dosed to achieve a postinfusion plasma fibrinogen level of at least 2.5 g/L in high-risk elective cardiac surgery patients 18 years or over with intraoperative bleeding reduced intraoperative blood loss (Bilecen et al. 2017).
The primary outcome of the study was intraoperative blood loss measured between intervention and closure of the chest when surgery ended. Secondary outcomes included the measured blood loss at 1, 3, 6,12 and 24 hours after the intervention; the proportion of patients who received transfusion; and number of units of RBCs, FFP or platelet concentrate.
The aim was to achieve surgical haemostasis after heparin reversal. It was the surgeons’ decision when to start the 5-minute bleeding time. Once patients had bled for 5 minutes they were entered into the treatment algorithm if they were bleeding >60 mL and <250 mL. The intervention was considered to have started on initiation of infusion of the study medication (Figure 1).
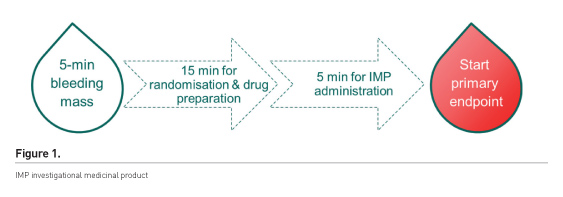
Over the four years of the trial, over 700 patients had complex cardiac surgery, of which 40% were eligible for the trial. Of the eligible patients, 43% did not agree to participate; 203 patients agreed to participate, of which 73 (36%) experienced no intraoperative bleeding and 10 were excluded for other reasons. We suggest that the high number of patients that experienced no intraoperative bleeding may be due to the Hawthorne effect—performing differently when being observed. This group of patients had very extensive surgical haemostasis before we started with the 5 minutes bleeding time, much longer than usual. The surgeons may have perceived that they were a better surgeon if they were not included in the trial, and also that after closure of the chest the microvascular bleeding was now the problem for the anaesthesiologist.
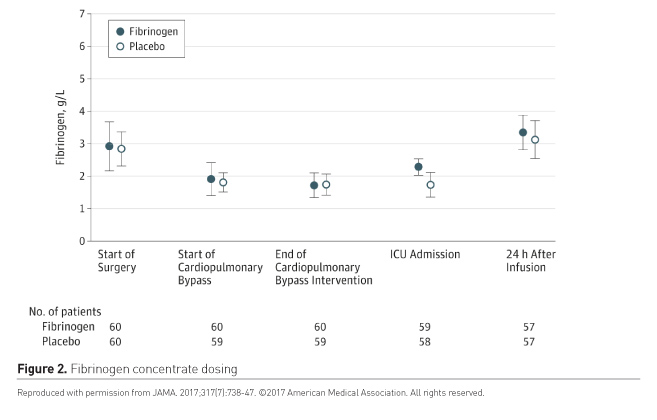
The patients were randomised to receive either the placebo or the intervention drug in doses between 60ml and 250ml. The fibrinogen doses were calculated based on plasma fibrinogen levels at the end of cardiopulmonary bypass measured using the Clauss method (Figure 2).
Results
Primary outcome
Among patients with intraoperative bleeding who received infusion of fibrinogen concentrate, compared with placebo, there was no significant difference in blood loss measured from the time of the fibrinogen infusion and chest closure (p = 0.19) (Table 1).
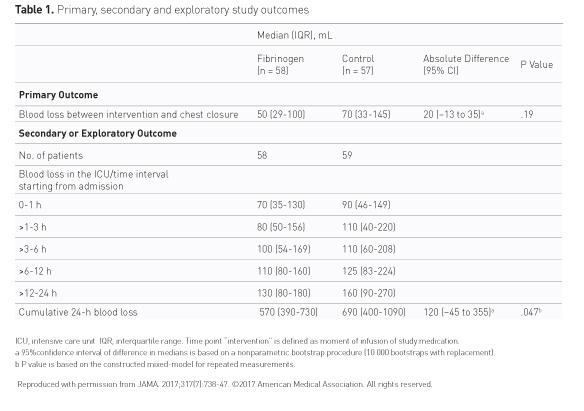
fibrinogen group
(median, 50 mL; IQR, 29-100 mL)
control group
(median, 70 mL; IQR, 33-145 mL)
absolute difference:
20 mL (95% CI, −13 to 35 mL)
Cumulative 24-hour blood loss was lower in the fibrinogen group compared with placebo (p = 0.047).
The duration of primary outcome collection was 4.2 minutes
(95% CI, 0.4-8.0 minutes) in the fibrinogen group and 8.7 minutes (95% CI,
5.2-12.1 minutes) in the control group.
However, other than these times do
suggest these were not the best and fastest surgeons ever. This is why the
primary endpoint was different than we expected. We expected that the moment
there is a bleeding patient everyone would wait for closure and this would lead
to a difference in blood loss in the patient and of course a difference in the
intraoperative time factor.
Secondary outcomes
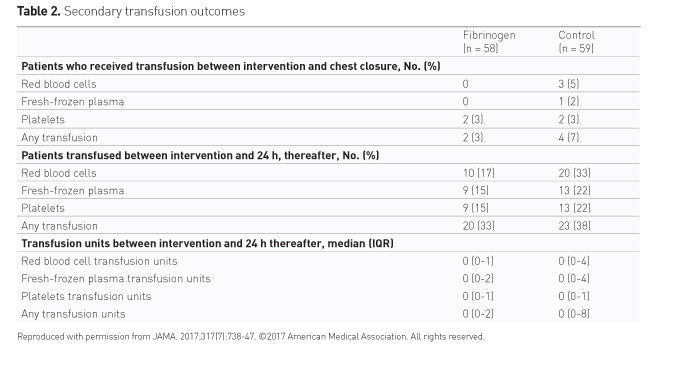
There were fewer patients in the fibrinogen group and fewer units of blood units used (Table 2). However, the study was not adequately powered to test the secondary outcomes. The percentage of reduction in transfusion is shown in Figure 3.
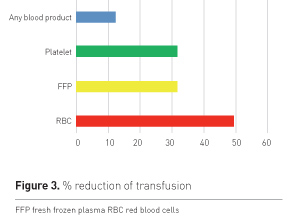
After surgery, a single patient (2%) in the fibrinogen group and 6 patients (10%) in the control group received additional fibrinogen concentrate. This confounds the effect on overall outcomes.
Procoagulants and antifibrinolytics use is shown in Table 3. The percentage of reduction is shown in Figure 4.
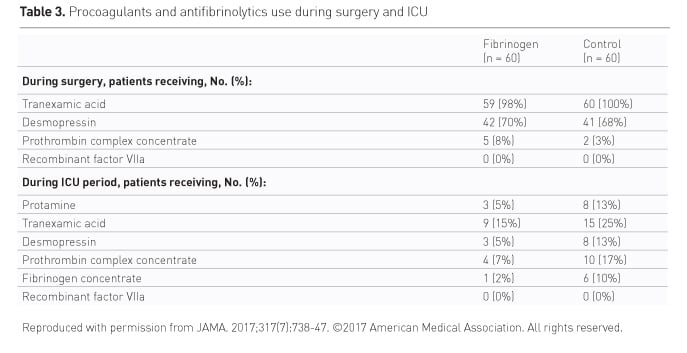
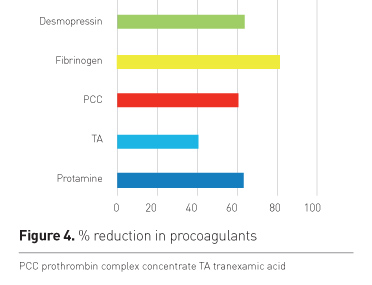
Clinical adverse events within 30 days
There were more adverse events in the fibrinogen group (Table 4). Two patients died, and 4 suffered a stroke. One patient in the control group suffered a stroke, and one a transient ischaemic attack. The trial was not designed to evaluate major adverse cardiac events and there was no screening for embolic risk aortic disease.
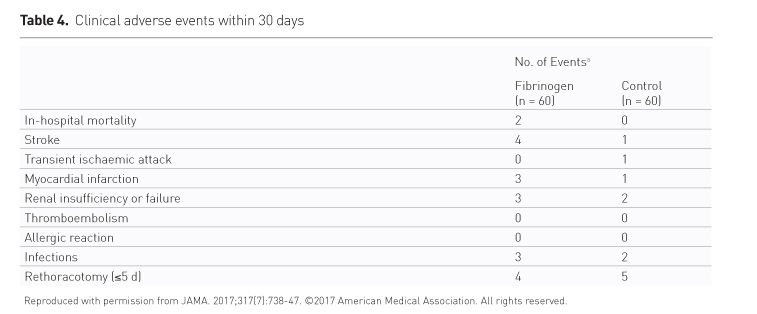
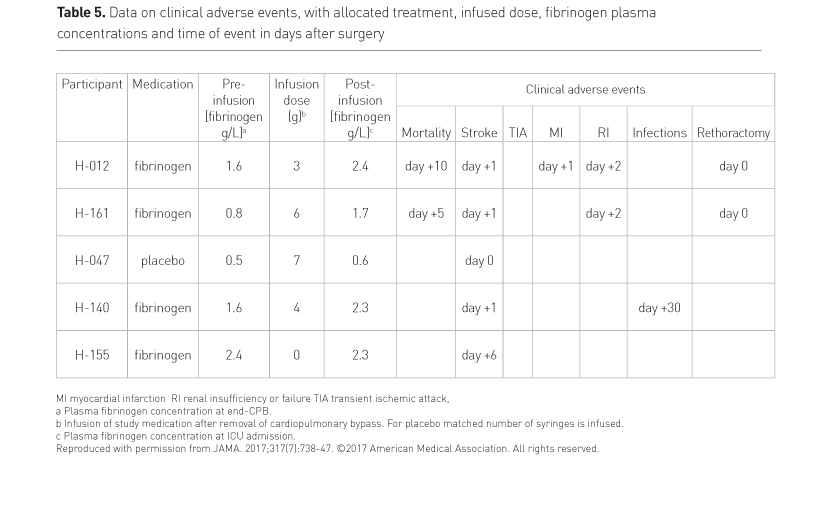
Almost 50% (9/19) of adverse events occurred in two patients (Table 5) (see p. VIII). Also one stroke occurred in a placebo patient with a fibrinogen level of 0.6g/l.
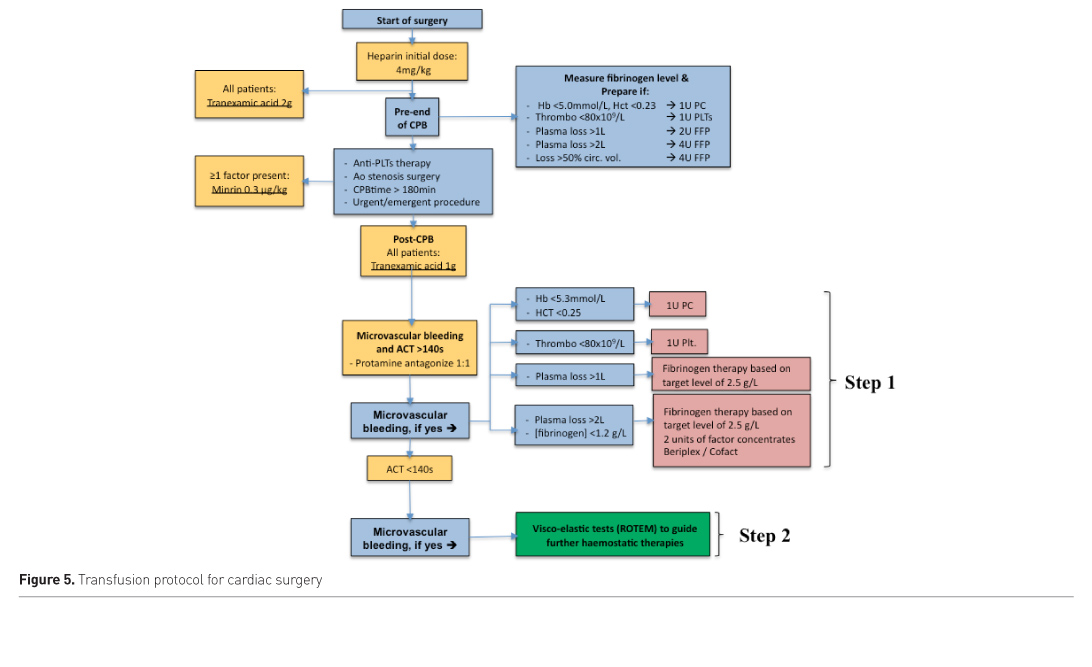
Transfusion protocol for cardiac surgery
At Isala Clinics we now use the following transfusion protocol (Figure 5). We conduct viscoelastic tests (ROTEM) to guide haemostatic therapies. Maegele and colleagues have provided a useful haemotherapy algorithm (Maegele et al. 2017).
Conclusion
Fibrinogen is effective after complex cardiac surgery in the
bleeding patient. Based on the current trial data, fibrinogen is recommended as
a first-line therapy with target plasma level 2.5g/L at the moment there is an
idea of microvascular bleeding in these patients. Both visco-elostometry as the
conventional Clauss method can be used to determine the level of fibrinogen at
the end of bypass surgery and to optimise further treatment.
We have the tools
and knowledge now to change transfusion management. But changing transfusion
management, as with any change, is a major behavioural process. You need to do
this together with your own multidisciplinary group, using a change strategy
such as the Kotter model (https://www.kotterinternational.com/8-stepsprocess-for-leading-change).
Conflict of Interest
Arno Nierich is the principal investigator for the fibrinogen concentrate trial at Isala Clinics. CSL Behring sponsored the study and donated the bottles of study medication.
Key Points
- Implement transfusion management with change strategy
- Multidisciplinary group essential
- Point-of-care and lab monitoring:
- Part of overcoming the ’blind spot’ of coagulation management in operating room and intensive care unit
- Additional tool in fine-tuning bleeding management
- Measure fibrinogen level by Clauss as first potential bleeding indication
- Fibrinogen is effective after complex cardiac surgery in the
bleeding patient: from rescue medication to first-line therapy with target
level 2.5 g/L in the bleeding patient
Abbreviations
CFC coagulation factor concentrates
FFP fresh frozen plasma
ICU intensive care unit
PBM patient blood management
RBC red blood cells
Supplement from CSL Behring in collaboration with ICU Management & Practice
References:
Bennett-Guerrero E, Zhao Y, O'Brien SM et al. (2010) Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA, 304(14): 1568-75.
Bilecen S, Peelen LM, Kalkman CJ et al. (2013) Fibrinogen concentrate therapy in complex cardiac surgery. J Cardiothorac Vasc Anesth, 27(1): 12-7.
Bilecen S, de Groot JA, Kalkman CJ et al. (2014) Effectiveness of a cardiac surgeryspecific transfusion protocol. Transfusion, 54(3): 708-16.
Bilecen S, de Groot JA, Kalkman CJ et al. (2017) Effect of fibrinogen concentrate on intraoperative blood loss among patients with intraoperative bleeding during highrisk cardiac surgery: a randomized clinical trial. JAMA, 317(7): 738-47.
Isbister JP, Shander A, Spahn DR et al. (2011) Adverse blood transfusion outcomes: establishing causation. Transfus Med Rev, 25(2):89-101.
Moraca RJ, Wanamaker KM, Bailey SH et al. (2011) Strategies and outcomes of cardiac surgery in Jehovah's Witnesses. J Card Surg, 26(2): 135-43.







