ICU Management & Practice, Volume 16 - Issue 3, 2016
Most patients with liver cirrhosis remain in a compensated stage for more than 10 years, regardless of the aetiology of the liver disease. The progression to decompensated cirrhosis is defined by the occurrence of a major complication such as ascites, variceal bleeding and/or hepatic encephalopathy. From here on most patients will not die because of a progressive, irreversible decrease in liver function, but because of a relatively sudden event that precipitates an acute deterioration in their clinical condition, a syndrome termed acute-on-chronic liver failure (ACLF). For many intensive care specialists, ACLF stands for a critically ill patient who is suffering from an intra- or extrahepatic acute insult with serious repercussions on both an existing chronic liver disease and on other organ functions. It also means that, as compared to the average intensive care unit (ICU) patient, the patient has an unusually high risk of death.
Concepts about cirrhosis have
evolved significantly in recent years, and major advances have been made in
defining the natural history of ACLF (for general reviews see Arroyo et al.
2016; Bernal et al. 2015; Sarin and Choudhury 2016). The syndrome is highly challenging
for intensivists and poses difficult questions related to the recognition of
precipitating factors, pathogenesis of extrahepatic organ failures, accurate
prognosis, medical management, evaluation for urgent liver transplantation
and finally the identification of those situations that may render intensive care
futile. The present appraisal will focus on recent insights and their
potential repercussions on the way intensivists should understand and manage
patients with ACLF.
Definition and Natural History of Acute-on-Chronic Liver Failure
There is no uncontested universal
definition for ACLF and the two most widely used definitions depend on the
origin of the hepatologists— West versus East (Arroyo et al 2015; Sarin et al.
2014). For the purpose of this text we will use the definition of the
European Association for the Study of the Liver – Chronic Liver Failure (EASL-CLIF)
Consortium, because extrahepatic organ failure(s) and short-term mortality are
central to the definition and therefore more closely mimic circumstances in
the ICU. This definition is based on a prospective, multicentre, observational
study (CANONIC study) of 1343 patients who were hospitalised for acute
decompensation of cirrhosis (Moreau et al 2013). ACLF is thus defined as a
specific syndrome comprising acute decompensation of cirrhosis (development
of ascites, variceal bleeding, hepatic encephalopathy and/or bacterial
infections), organ failure and high short-term mortality (by definition 28-day
mortality rate ≥15%) (Arroyo et al 2015).
Based on the chronic liver
failure (CLIF) Acute-on-Chronic Liver Failure in
Cirrhosis (CANONIC) study a new grading system for severity of ACLF (grade 0
to 4) has been introduced built on a modified Sequential Organ Failure
Assessment (SOFA) score (Tables 1 and 2). This new grading system is
proving useful to diagnose the condition, to study the natural history of
ACLF, to stratify patients in interventional trials and for prognostication
(Gustot et al 2015; Silva et al. 2015; Shi et al. 2016).
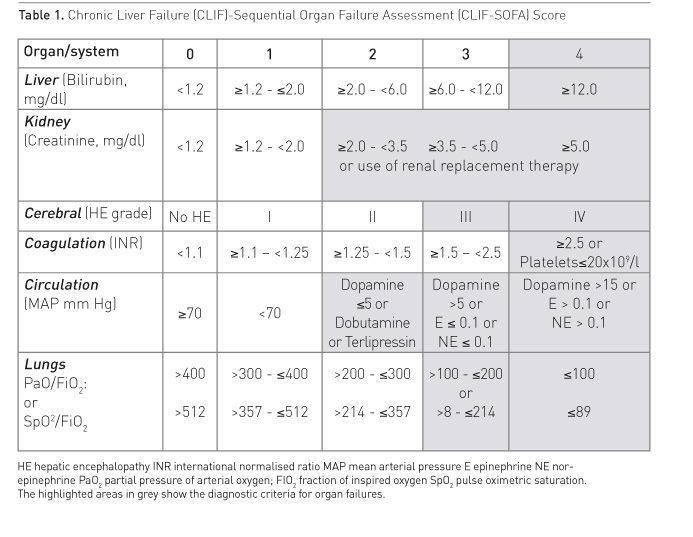
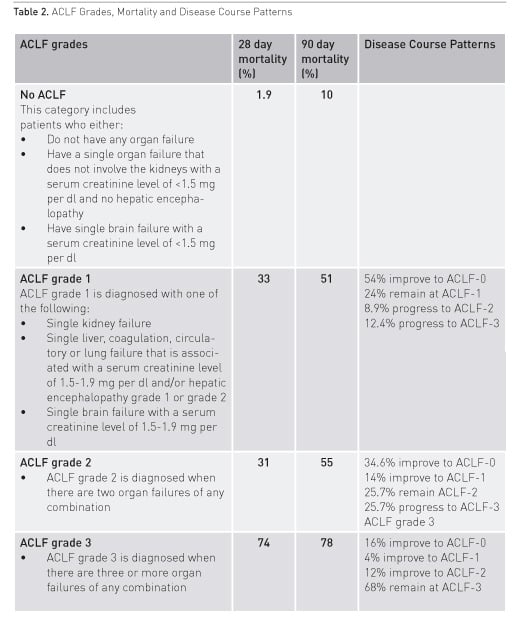
In the CANONIC study the prevalence of ACLF in patients presenting to the hospital with acute decompensation of cirrhosis was 31%. Twenty-three percent had ACLF at the time of admission and another 11% developed ACLF during hospitalisation. Twenty-four percent of the patients required care in the ICU with one in three not fulfilling criteria for ACLF at the time of admission to the ICU. A similar prevalence ranging from 24 to 34% has been reported in other large studies from China, North America and Scandinavia (Li et al 2016; Bajaj et al. 2014a; Sargenti et al. 2015). Almost half of the patients with ACLF did not have a prior history of acute decompensation, or had developed the first decompensating event within the three months prior to the diagnosis of ACLF. This observation is relevant to the extent that ACLF is not necessarily the final event in a progressive course of decompensating liver disease, but may occur at any point in time after diagnosis of cirrhotic liver disease.
The clinical course of the condition is very dynamic. One study observed resolution of ACLF in 42.5% of patients across all grades of ACLF, 53.5% in ACLF-1, 34.6% in ACLF-2 and 16% in ACLF-3 (Table 1) (Gustot et al. 2015). In the CANONIC study the overall 28-day and 90-day mortality rates for patients with ACLF, who did not undergo liver transplantation, were 32.8% and 51.2%. Similar rates have been reported in other studies (Li et al. 2016). These mortality rates are clearly different from those in patients with acute decompensation of liver cirrhosis but not fulfilling criteria for ACLF (1.9% and 9.3%, respectively). The most frequent cause of death in patients with ACLF was multiple organ failure without septic or hypovolaemic shock (40%), followed by septic shock in approximately 25% of cases. The aetiology of cirrhosis does not seem to be determinant of outcome, but patients with gastrointestinal haemorrhage as a precipitating factor do better than patients who were not bleeding at admission (McPhail et al. 2014).
It is often assumed that acute decompensation of liver function is triggered by a clinically identifiable, precipitating event. The trigger may have a hepatic origin, such as drug-induced liver injury, viral or ischaemic hepatitis, liver surgery or undue alcohol consumption. It can also have an extrahepatic origin such as acute bacterial infection, major surgery or paracentesis. Interestingly, in the CANONIC study, in 43.6% of the patients with ACLF, no precipitating event could be identified (Moreau et al. 2013). This observation underscores the fact that in the majority of patients we are not yet able to diagnose the pathogenetic mechanism leading to acute decompensation. Acute bacterial infection was the most frequent precipitating event in 33% of the patients (Moreau et al. 2013).
See Also: Liver Intensive Care
Prevalence and Pathogenesis of Organ Dysfunctions Associated With ACLF
Organ dysfunction or failure is highly prevalent in ACLF. Hepatic, renal, cerebral, coagulation and circulatory dysfunctions are well known, but important derangements in the function of the heart, immune system, adrenal glands and muscle have been also well documented. In ACLF patients in the CANONIC study kidney failure (56%) was the most frequent organ failure followed by liver failure (44%), coagulation (28%), cerebral (24%), circulation (17%) and lung failure with 9%. The number of failing organs correlates with increasing white cell count and C-reactive protein (CRP) levels (Jalan and Williams 2002).
Two pathogenetic mechanisms seem to be important drivers of both intra- and extrahepatic organ dysfunction: systemic inflammation and dysbiosis of the microbiome (Bernardi M. et al. 2015). Systemic inflammation may be induced by bacterial pathogen-associated molecular patterns (PAMPs) or by virulence factors produced by bacteria. Patients with cirrhosis have increased permeability of the gut related to portal hypertension, inflammation- mediated damage to the gut barrier and altered gut flora. The result is increased translocation of particularly Gram-negative bacteria, PAMPs or virulence factors from the intestinal lumen to the systemic circulation. The prevalence of translocation of enteric organisms to mesenteric lymph nodes in cirrhotic patients is significantly increased according to the Child- Pugh classification: 3.4% in Child A, 8.1% in Child B and 30.8% in Child C patients (Cirera et al. 2001). Systemic inflammation may also be induced by ongoing necrosis of hepatocytes or damage to the extracellular matrix caused by alcohol, viral disease or any other aetiopathogenetic mechanisms of cirrhosis. In this case the molecules inducing inflammation are called damage-associated molecular patterns (DAMPs). How inflammation contributes to organ dysfunction in ACLF has not yet been fully elucidated. Besides the well-described severe immune dysfunction associated with cirrhosis with increased susceptibility to infection, the following concepts are likely to be important (Verbeke et al. 2011):
- The effects caused by
immunopathology, a term that describes the potential negative impact of an
excessive immune response (Iwasaki and Medzhitov 2015). Either PAMPs or DAMPs
can cause immunopathology that in turn may cause organ dysfunction. In this
case defence mechanisms directed at controlling infection or immunopathology
are insufficient. This is the likely mechanism in ACLF precipitated by acute
bacterial infection or severe alcoholic hepatitis.
- Failed tolerance, a concept that describes the incapacity to develop tolerance mechanisms to persistent infection–mediated inflammation (Medzhitov et al 2012). In this case persistent ‘low-grade’ systemic exposure to PAMPs or DAMPs may be the reason for ongoing ‘sterile’ inflammation for which no tolerance can be developed. This concept provides an array of potential new therapeutic targets aimed at increasing tolerance.
Recent evidence points to gut dysbiosis as a second important pathogenetic driver of organ dysfunction in ACLF (Bajaj et al. 2014b; Chen et al. 2015; Rai et al. 2014). Several factors contribute to altered microbiota in cirrhosis, including increased intestinal permeability, abnormal small intestinal motility, impaired antimicrobial defence, small intestinal bacterial overgrowth, decreased bile acid production and compromised enterohepatic circulation (Rai et al. 2014). In stable cirrhosis there is a clear change in diversity and composition of gut microbiota with progressive dysbiosis in the setting of decompensation. Similar changes have been reported in ACLF. In a recent trial a relative abundance of Pasteurellacae was an independent predictor for mortality and, interestingly, the use of antibiotics had only moderate impact on the gut flora (Chen et al. 2015). Robust correlations were also observed between specific bacterial families and inflammatory cytokines such as interleukin-6 and TNF-alpha. A clear mechanistic link between pathogenic colonic mucosal microbiota and poor cognition has been demonstrated for hepatic encephalopathy (Rai et al. 2014; Bajaj et al. 2012). Remarkably, treatment with lactulose in patients with hepatic encephalopathy did not change faecal flora composition. It remains unclear how gut dysbiosis contributes to organ dysfunction. Current findings suggest that relative gut overgrowth of one type of bacteria or metabolites of certain bacteria species can contribute to inflammation and thereby to organ dysfunction.
Potential New
Therapeutic Approaches
In specific situations early treatment of precipitating events such as alcoholic hepatitis with steroids or reactivation of hepatitis B with antivirals can reduce mortality. However, and for the most part, medical management of organ failure in ACLF remains supportive. Randomised trials with extracorporeal liver support systems aimed at blood purification did not result in survival benefits (Banares et al. 2013; Kribben et al. 2012).
A recent observational study reported improved clinical outcome with plasma exchange in hepatitis B-related ACLF (Chen 2016). High hopes are placed in regenerative therapy of cirrhosis including the use of growth factors, the combination of G-CSF and erythropoietin, hepatocyte and stem cell transplantation (King et al. 2015; Kedarisetty et al. 2015, Shiota and Itaba 2016; Duan et al. 2013; Garg et al. 2012; Zekri et al. 2015). Granulocytecolony stimulating factor (G-CSF) therapy in ACLF reduced organ dysfunction and improved survival (Chavez-Tapia et al. 2015). It is unclear if the positive results obtained in randomised trials with administration of G-SCF in ACLF patients will be applicable in more severe forms of ACLF in the ICU (Duan et al. 2013; Garg et al. 2012).
Prognosis, Futility and Eligibility for Liver Transplantation
Many intensivists take a reserved attitude towards the admission of ACLF patients because of the dim prognosis of the syndrome. However, several new facts have emerged in recent years that defend a change in attitude and justify a full evaluation for transplant for every patient with ACLF admitted to the ICU. First, new data show that liver fibrosis and even cirrhosis are potentially reversible if the underlying cause is removed, with significant improvement in longterm survival (Ramachandran 2015). Second, the outcome of ACLF in the ICU has improved considerably. In expert ICUs survival of patients with cirrhosis and organ failure improved from 40% in the year 2000 to 63% in the year 2010 (McPhail et al. 2014). Similarly, ICU mortality of cirrhotic patients with septic shock has decreased from 74% in 1998 to 65.5% in 2010 (Galbois et al. 2014). Third, the course of the disease is very dynamic with resolution or improvement of ACLF in 4.2% of patients. Eighty-one precent reach their final ACLF grade at one week after admission, and it is now clear that for most patients prognostication will be considerably more accurate if done towards the end of the first week of ICU stay (Gustot et al. 2015). Fourth, prognostication for these patients has improved. New scoring systems, such as the Chronic Liver Failure Consortium Acuteon- Chronic Liver Failure score (CLIF-C ACLF) score that incorporates a modified SOFA-score (CLIF-Organ Failure [OF] score), age and white blood cell count can be calculated on a daily base in order to monitor evolution/resolution of ACLF and provide a significantly better estimate of risk for mortality than the model for end-stage liver disease (MELD) or Child-Pugh score (Jalan et al. 2014).
Considering the above, indiscriminate refusal of ICU admission of ACLF patients is not acceptable any more, since no specific group of patients can be identified at the time of diagnosis for which medical ICU treatment may be considered futile. However, intensivists also need to acknowledge that patients with four or more organ failures or a CLIF-C ACLF score > 64 after one week of ICU care have 28-day mortality rates in the range of 90 to 100%. If ineligible for transplantation withdrawal of care is a reasonable option for these patients.
Liver transplantation in ACLF is controversial and fraught with uncertainties regarding case selection and timing (Pamecha et al. 2015; Reddy et al. 2015). Only 15-25 % of patients are actually transplanted (Gustot et al. 2015, Finkenstedt et al. 2013). Recent series have reported encouraging results with 1- and 5-year survival of 80-90% (Finkenstedt et al. 2013; Chan et al. 2009). Even patients with ACLF-3 may expect a 1-year survival probability of 78% (Gustot et al. 2015) .
Summary
Major progress has been made in defining the natural history and prognosis of ACLF. Regenerative therapies and liver transplantation in selected cases hold promise for the future.
Conflict of Interest
Philippe Meersseman and Alexander Wilmer declare that they have no conflict of interest.
Abbreviations
ACLF acute-on-chronic liver failure
CANONIC CLIF Acute-on-Chronic Liver Failure in Cirrhosis
CLIF chronic liver failure
CLIF-C Chronic Liver Failure Consortium
CRP c-reactive protein
DAMP damage-associated molecular patterns
G-CSF Granulocyte-colony stimulating factor
ICU intensive care unit
MELD model for end-stage liver disease
OF organ failure
PAMP pathogen-associated molecular patterns
SOFA sequential organ failure assessment
References:
Arroyo V, Moreau R, Jalan R
et al. (2015) Acute-on-chronic liver failure: a new syndrome that will
re-classify cirrhosis. J Hepatol, 62(1 Suppl): S131-43.
PubMed ↗
Arroyo V, Moreau R, Kamath PS et al. (2016) Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers, 2: 16041.
PubMed ↗
Bajaj JS, Hylemon PB, Ridlon JM et al. (2012) Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrontest Liver Physiol, 303 (6): G675-85.
PubMed ↗
Bajaj JS, O'Leary JG, Reddy KR et al. (2014a) Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology, 60(1): 250-6.
PubMed ↗
Bajaj JS, Heuman DM, Hylemon PB et al. (2014b) Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol, 60(5): 940-7.
PubMed ↗
Banares R, Nevens F, Larsen FS et al. (2013) Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELEF trial. Hepatology, 57(3): 1153-62.
PubMed ↗
Bernal W, Jalan R, Quaglia A et al. (2015) Acute-on-chronic liver failure. Lancet, 386(10003): 1576-87.
PubMed ↗
Bernardi M, Moreau R, Angeli P et al. (2015) Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol, 63(5): 1272-84.
PubMed ↗
Chan AC, Fan ST, Lo CM et al. (2009) Liver transplantation for acute-on-chronic liver failure. Hepatol Int, 3(4): 571-81.
PubMed ↗
Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ et al. (2015) Granulocyte-colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Ann Hepatol, 14(5): 631-41.
PubMed ↗
Chen Y, Guo J, Qian G et al. (2015) Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J Gastroenterol Hepatol, 30(9): 1429-37.
PubMed ↗
Chen JJ, Huang JR, Yang Q et al. (2016) Plasma-exchange-centred artificial liver support system in hepatitis B virus-related acute-on-chronic liver failure: a nationwide prospective multicentre study in China. Hepatobiliary Pancreat Dis Int, 15(3): 275-81.
PubMed ↗
Cirera I, Bauer TM. Navasa M et al. (2001) Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol, 34(1): 32-7.
PubMed ↗
Duan XZ, Liu FF, Tong JJ et al. (2013) Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic-liver failure. World J Gastroenterol, 19(7): 1104-10.
PubMed ↗
Finkenstedt A, Nachbaur K, Zoller H et al. (2013) Acute-on-chronic liver failure: excellent outcomes after liver transplantation but high mortality on the waiting list. Liver Transpl, 19(8): 879-86.
PubMed ↗
Galbois A, Aegerter P, Martel-Samb P et al. (2014) Improved prognosis of septic shock in patients with cirrhosis: a multicenter study. Crit Care Med, 42(7): 1666-75.
PubMed ↗
Garg V, Garg H, Khan A et al. (2012) Granulocyte-colony stimulating factor mobilizes CD34+ cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology, 142: 505-12.
PubMed ↗
Gustot T, Fernandez J, Garcia E et al. (2015) Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology, 62(1): 243-52.
PubMed ↗
Iwasaki A, Medzhitov R (2015) Control of adaptive immunity by the innate immune system. Nat Immunol, 16(4): 343-53.
PubMed ↗
Jalan R, Williams R (2002) Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif, 20(3): 252-61.
PubMed ↗
Jalan R, Saliba F, Pavesi M et al. (2014) Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol, 61: 1038-47.
PubMed ↗
Kedarisetty CK, Anand L, Bhardwaj A et al. (2015) Combination of granulocyte colony-stimulating factor and erythropoietin improves outcomes of patients with decompensated cirrhosis. Gastroenterology, 148(7): 1362-70.
PubMed ↗
King A, Barton D, Beard HA et al. (2015) Repeated AutoLogous infusions of stem cells in cirrhosis (REALISTIC): a multicentre, phase II, open-label, randomised controlled trial of repeated autologous infusions of granulocyte colony-stimulating factor (GCSF) mobilised CD133+ bone marrow stem cells in patients with cirrhosis. A study protocol for a randomised controlled trial. BMJ Open, 5(3): e007700.
PubMed ↗
Kribben A, Gerken G, Haag S et al. (2012) Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology, 142(4): 782-789.e3.
PubMed ↗
Li H, Chen LY, Zhang NN et al. (2016) Characteristics, diagnosis and prognosis of acute-on-chronic liver failure in cirrhosis associated to Hepatitis B. Sci Rep, 6: 25487.
PubMed ↗
McPhail MJ, Shawcross DL, Abeles RD et al. (2015) Increased survival for patients with cirrhosis and organ failure in liver intensive care and validation of the chronic liver failure-sequential organ failure scoring system. Clin Gastroenterol Hepatol, 13(7): 1353-1360.e8.
PubMed ↗
Medzhitov R, Schneider DS, Soares MP (2012) Disease tolerance as a defence strategy. Science, 335(6071): 936-41.
PubMed ↗
Moreau R, Jalan R, Gines P et al. (2013) Acute-on-chronic liver failure s a distinct
syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology, 144(7): 1426-37.
PubMed ↗
Pamecha V, Kumar S, Bharathy KG (2015) Liver transplantation in acute on chronic liver failure: challenges and an algorithm for patient selection and management. Hepatol Int, 9(4): 534-42.
PubMed ↗
Rai R, Saraswat VA, Dhiman RK (2014) Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol, 5(Suppl 1): S29-36.
PubMed ↗
Ramachandran P, Iredale JP, Fallowfield JA (2015) Resolution of liver fibrosis: basic mechanisms and clinical relevance. Semin Liver Dis, 35(02): 119-31.
PubMed ↗
Reddy MS, Rajalingam R, Rela M (2015) Liver transplantation in acute-on-chronic liver failure: lessons learnt from acute liver failure setting. Hepatol Int, 9(4): 508-13.
PubMed ↗
Sargenti K, Prytz H, Nilsson E et al. (2015) Predictors of mortality among patients with compensated and decompensated liver cirrhosis: the role of bacterial infections and infection-related acute-on-chronic liver failure. Scand J Gastroenterol, 50(7): 875-83.
PubMed ↗
Sarin SK, Kedarisetty CK, Abbas Z et al. (2014) Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int, 8(4): 453-71.
PubMed ↗
Sarin SK, Choudhury A (2016) Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol, 13(3): 131-49.
PubMed ↗
Shi Y, Shu Z, Sun W et al. (2016) Risk stratification of decompensated cirrhosis patients by the CLIF Consortium scores: a classification and regression tree analysis. Hepatol Res, doi: 10.1111/hepr.12751 [Epub ahead of print]
PubMed ↗
Shiota G, Itaba N (2016) Progress in stem cell-based therapy for liver disease. Hepatol Res, doi: 10.1111/hepr.12747 [Epub ahead of print]
PubMed ↗
Silva PE, Fayad L, Lazzarotto C et al. (2015) Single-centre validation of the EASL-CLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int, 35(5): 1516-23.
PubMed ↗
Verbeke L, Nevens F, Laleman W (2011) Bench-to-beside review: acute-on-chronic liver failure - linking the gut, liver and systemic circulation. Crit Care, 15(5): 233.
PubMed ↗
Zekri AR, Salama H, Medhat E et al. (2015) The impact of repeated autologous infusion of hematopoietic stem cells in patients with liver insufficiency. Stem Cell Res, 6: 118.
PubMed ↗








