ICU Management & Practice, Volume 24 - Issue 4, 2024
A review of recent evidence on early mobilisation and rehabilitation and what remains to be defined.
Introduction
Critical illnesses encompass a broad spectrum of pathologies that require support for different organs. This often leads to prolonged bed rest and secondary immobilisation, which ultimately fosters the development of Intensive Care Unit-acquired Weakness (ICU-AW). ICU-AW is the onset of muscle weakness detected in critically ill patients without a plausible cause other than critical illness, which can extend beyond hospital discharge (Stevens et al. 2009; Vanhorebeek et al. 2020). It is characterised by generalised muscle weakness with a predominance of proximal and symmetrical muscle involvement (Latronico et al. 2017; Vanhorebeek et al. 2020). ICU-AW harms patient's recovery from critical illness, leads to a deterioration in the quality of life, and recovery may be incomplete. Its prevalence ranges between 25% and 80% of ICU patients (Kho and Connolly 2023).
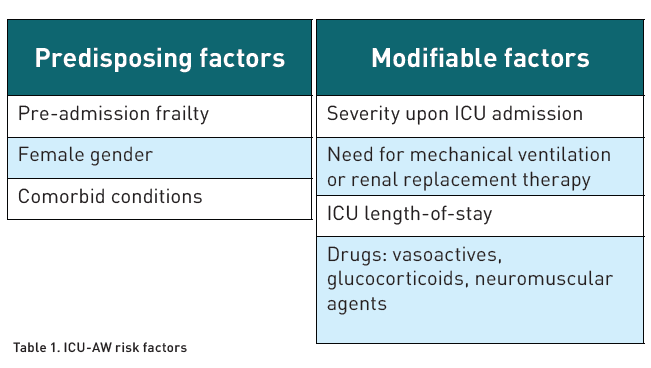
Different studies have classified ICU-AW into myopathy, polyneuropathy or a combination of both (Stevens et al. 2009). Numerous risk factors associated with ICU-AW have been described (Table 1) (Yang et al. 2018; Yang et al. 2022).
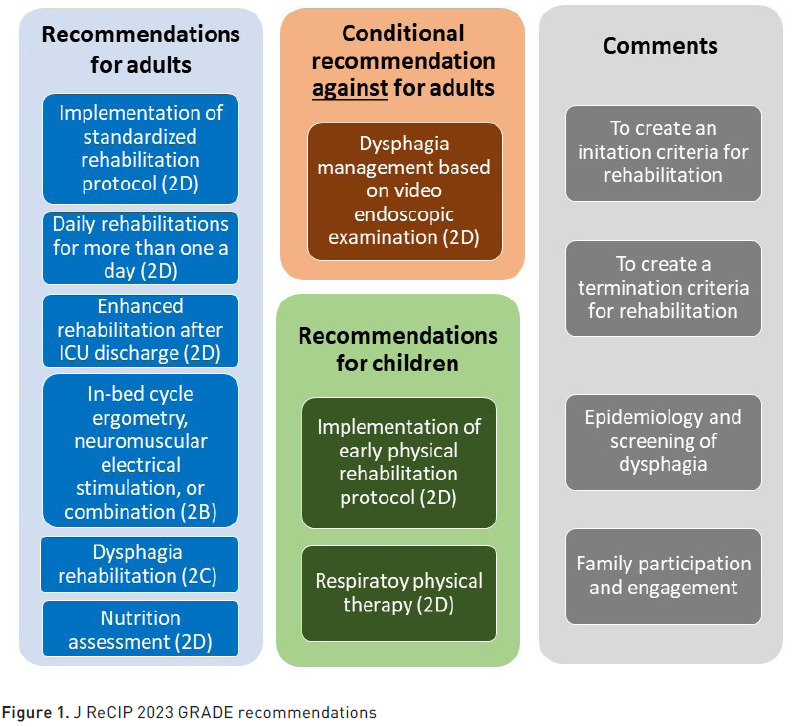
Despite the increase in research, studies focused on rehabilitation remain limited due to the absence of a standardised and agreed-upon set of outcomes (Kirkham and Williamson 2022). A systematic review (Lang et al. 2020) evaluated the quality and content of existing clinical guidelines. Despite the heterogeneity of the included publications and significant gaps in the evidence-based literature, it was demonstrated that there is an agreement on the principle of applying early mobilisation. The main areas for improving methodological quality and guideline information were as follows: consistent involvement of patients and families in the guideline development process, detailed evaluation of the quality of existing literature, external review, provision of an updated procedure, and review of existing literature on barriers and facilitators. It is worth highlighting the attempt of the Japanese Society of Intensive Care Medicine to provide standardised rehabilitation guidelines (Unoki et al. 2023) based on ten GRADE (Grading of Recommendations Assessment, Development and Evaluation) recommendations and four comments. The key points are summarised in Figure 1.
Defining Early Mobilisation
Mobilisation and rehabilitation activities overlap. The terms are often used interchangeably, although there are notable differences in the therapeutic basis. Mobility is "the process of moving oneself and changing and maintaining positions" (Bussmann and Stam 1998). Any member of the critical care team can perform mobility. In contrast, rehabilitation is "a set of interventions designed to optimise functioning and reduce disability in individuals with health conditions in interaction with their environment" (World Health Organization 2023). Rehabilitation interventions reflect individualised goals to address patients' needs. Rehabilitation professionals such as physiotherapists and occupational therapists have specialised skill sets with specific knowledge to assess deficits. That is, rehabilitation requires a high level of teamwork. Its successful application requires continuous interprofessional collaboration and communication, which can be enhanced with interprofessional rounds, standardised protocols, and shared mobilisation goals (Dubb et al. 2016; Lang et al. 2020).
How Early is Early Mobilisation Recommended?
Studies differ in the timing of the initiation of rehabilitation, which appears to have implications for outcomes. Studies where early mobilisation was started within 24 to 72 hours after ICU admission (Dong et al. 2014; Liu et al. 2022; Schaller et al. 2016) present more favourable outcomes than those where rehabilitation was delayed until the fifth to seventh day of admission, with no differences in hospital stay or functional status (Walsh et al. 2015; Wright et al. 2018).
When considering when to start early mobilisation, we must also acknowledge each patient's characteristics: one size does not fit all (Fuest et al. 2023). In this case, grouping patients according to specific traits allows for optimised treatment. For example, the subset of patients most likely to benefit from physical rehabilitation appears to be those with a prolonged ICU stay (Waldauf et al. 2020). However, patients with greater severity are more likely to suffer ICU-acquired complications (Vanhorebeek et al. 2020). Early evidence indicates that these severe and frail patients can still benefit from achieving higher mobility levels at ICU discharge.
Duration of the Session
Several reasons influence the duration of mobilisation: (1) patient-related, (2) provider-related, and (3) organisational factors. Patient-related factors are probably the most important: the intrinsic possibility and capacity for mobilisation out of bed depend on the pre-admission status and the current impact of the illness. Provider-related factors include workload, individual motivation or attitude towards mobilisation, and training. Organisational factors include the culture towards mobilisation (e.g., the existence of mobilisation teams) and the existence of standard operating procedures or local protocols.
The duration of mobilisation sessions in critically ill patients has not been extensively studied. Two published studies (Lorenz et al. 2023; Schumann et al. 2020) set the limit at more or less than 40 minutes with favourable results regarding the preservation of functionality. The results suggested that longer mobilisation durations could help preserve the functionality of critically ill patients who survive the ICU stay (improvement in functional status and greater independence at ICU discharge: 96% versus 44%; p < 0.001). However, the maximum mobilisation achieved was the most important of all mobilisation parameters influencing the outcome. Observing subgroups by the level of mobilisation in patients with the highest level, the duration of mobilisation of more than 40 minutes ceased to be statistically significant.
The interaction between different components of mobilisation remains complex, but what seems clear is that a high dose of mobilisation therapy was associated with better functional outcomes, reduced mortality, and shorter stays—both in ICU and hospital (Scheffenbichler et al. 2021; Watanabe et al. 2021). Paton et al. (2024) demonstrated that higher levels of mobilisation measured by the ICU Mobility Score (IMS) produced better long-term outcomes with a positive impact on both functional status and perceived quality of life. Fuest et al. (2023) confirmed that in severely frail patients, the maximum level of the Surgical ICU Optimal Mobilisation Score (SOMS) achieved was the most influential factor in home discharge. In contrast, in young, traumatised patients, a higher level was not associated with a higher probability of home discharge. Therefore, a uniform mobilisation approach targeting higher therapy levels does not seem helpful in the heterogeneous group of critically ill patients. Finally, the recently published TEAM trial (Hodgson et al. 2022) showed no benefit from more prolonged and intense active mobilisation (120 additional minutes per day) on long-term outcomes, showing a higher incidence of adverse events during the intervention. An individualised approach is needed.
Number of Sessions
A recent systematic review reported how using a basic definition of usual care dose impacted key outcomes (Wang et al. 2022). If usual care was provided less than five days a week, the effect of rehabilitation interventions was amplified with a reduction in mechanical ventilation (MV) duration by 16 days, ICU stay by 18.7 days, and hospital stay by 24 days. In contrast, if usual care was provided five days/week or more, there were no differences in the duration of MV, and the differences in ICU and hospital stay were minor.
Contrary to what we might think, it has also been described (Bernhardt et al. 2015; Greening et al. 2014) that very early, intense, and high-dose mobilisation does not always have the best results in some patient cohorts. ICU-acquired weakness has muscular and nervous system characteristics that may limit the response to treatment.
Rehabilitation Strategies
The exercise performed during rehabilitation can be classified as passive, assisted, or active. Other research groups classify it into functional exercises (sitting, walking, rolling) and non-functional exercises, which include a range of motion, whether active or passive, neuromuscular electrical stimulation, and cycle ergometry (Nadeau et al. 2013; Wang et al. 2022). Studies show that passive mobilisation (Vollenweider et al. 2022) presents only a positive trend in sedated and ventilated patients concerning muscle structure, microcirculation, inflammation, and immune system factors. However, apparent efficacy could not be demonstrated. Different strategies and equipment can help with assisted and active mobilisation, and differences in the type of intervention can influence the demonstration of clinically significant differences (Hodgson et al. 2021).
Higher levels of mobilisation require patient participation. Consequently, physical rehabilitation is more effective when coordinated with proper management of analgosedation. Once again, interprofessional teamwork is key to coordinating the daily management of critically ill patients and rehabilitation strategies; exquisite coordination between doctors, nurses, physiotherapists, and occupational therapists is required. Their combined experience can be helpful in specific rounds for patients with complex needs, discussing recovery challenges, or setting care goals.
Current recommendations are for a gradual progression of functional exercises for at least five days a week (Wang et al. 2022). However, careful monitoring of load and rest is necessary to ensure recovery between sessions.
Beyond Rehabilitation
Other co-interventions should be considered when implementing rehabilitation. It is necessary to tailor the energy needs to the exercise and the stage of critical illness in which the patient is. Adequate nutrition management will provide the required nutrients for optimal muscle performance, minimise the effects of protein catabolism in the late inflammatory phase, and avoid overfeeding (Liu et al. 2024).
A proposed comprehensive strategy (De Man et al. 2024; Yébenes et al. 2024) involves (1) a detailed anamnesis and an adequate initial nutritional assessment to establish a medical and nutritional therapy according to the needs and characteristics of each patient; (2) a safe transition between nutritional therapy routes and between care units, with the primary objective of preserving lean mass in critically ill patients, considering metabolic factors, adequate protein intake, and muscle stimulation; (3) continuous monitoring due to the lack of precise tools to calculate nutritional efficiency in critically ill patients; and (4) a multidisciplinary approach. Such a comprehensive strategy can make a significant difference in the functional recovery of critically ill patients.
Regarding optimising the patient's nutritional aspect, swallowing function should be evaluated appropriately during ICU admission. It should be noted that the exact frequency of dysphagia in critically ill ICU patients remains uncertain due to the lack of a standardised approach. Due to variations in practices and dietary cultures in different countries, various screening methods for dysphagia have been devised, and an international standardisation has not been established. Additionally, although patients may swallow voluntarily, they may experience silent aspiration, making it necessary to combine several screening methods to determine the presence of dysphagia.
In critically ill patients, swallowing function is often impaired due to interventions such as endotracheal tube placement, tracheostomy, and surgical procedures. Older adults may have pre-existing dysphagia due to comorbidities and ageing. Dysphagia can also influence oral intake restrictions, dietary method changes, decisions regarding home discharge, and prognosis. Therefore, screening methods should ideally be easily performed at the bedside without special equipment. These methods must demonstrate high validity, reliability, sensitivity, and specificity and should be compared with reference techniques like video fluoroscopic swallowing studies or fibreoptic endoscopic evaluation of swallowing. Therefore, a combined assessment with a clinical review and endoscopic evaluation, which allows for greater diagnostic accuracy, is probably the correct approach, directing appropriate rehabilitation. Swallowing function rehabilitation should optimise sensory and motor functions, encompassing swallowing, cough efficacy, smell, and communication (Zaga et al. 2024).
How Do We Measure Outcomes?
Measuring outcomes is an essential part of the process, and the way to do it varies between studies. First, gathering information about the patient's functional status is crucial, affecting recovery goals after ICU admission (Muscedere et al. 2017). Additionally, a routine evaluation will help to adjust the rehabilitative treatment to the patient's situation and facilitate the transition at the patient's care level, ensuring continuity in the process and avoiding delays in recovery.
One of the challenges in selecting outcome measures for rehabilitation trials is the lack of reliable and validated measures to evaluate outcomes important to patients. For example, the EQ-5D is considered the most promising tool for measuring health-related quality of life. It is regularly used but has not been rigorously validated in the critically ill population (Lau et al. 2022). There is a lack of consensus on the appropriate timeframe for evaluating outcomes after rehabilitation and mobilisation interventions (Herridge and Azoulay 2023; Kho et al. 2023). It is essential to highlight that in qualitative research, patients describe an evolution of recovery priorities that differ over time (Scheunemann et al. 2020).
Barriers to Implementation
Physical rehabilitation is usually safe (Paton et al. 2024). However, two recent randomised controlled trials (Hodgson et al. 2022; Patel et al. 2023) reported increased adverse events. In particular, the reported events mainly consisted of temporary cardiorespiratory changes that occurred infrequently (<1% of 696 sessions) and rarely caused patient harm (0.1% of all patients). A recent meta-analysis comparing physical rehabilitation with usual care found no effects on the rate of adverse events [3% (693 events in 23,395 sessions); RR 1.09; 95% CI 0.69-1.74] or mortality [RR 0.98; 95% CI: 0.87-1.12] (Paton et al. 2023).
One of the first barriers we encounter when initiating early mobilisation is the haemodynamic instability that patients may present in the first days of admission, the need for deep sedation, or an altered level of consciousness. Regarding haemodynamic instability, the literature includes some studies on the dose of vasopressors considered safe for mobilisation without consensus (Lindholz et al. 2022; Yang et al. 2021). It is suggested that doses below 0.2 mcg/kg/min may be safe for mobilising patients.
Another barrier is patient safety concerns due to multiple catheters, tubes, and drains. Several articles have demonstrated that rehabilitating critically ill patients is generally safe (Adler and Malone 2012). Moreover, one of the leading causes of fear among staff is lack of training. A multiday protocol and ongoing training can significantly eliminate these barriers and provide security to healthcare personnel.
Patients recognise the importance of physical rehabilitation but often express it as a significant obstacle. Good communication and care consistency can foster patient confidence and participation (Van Willigen et al. 2020). Additionally, structured exercise plans that consider personal care, family visits, individual needs, and rest can reduce fatigue. A qualitative systematic review (Goddard et al. 2024) studied survivors' perceptions, opinions, and experiences on physical recovery and rehabilitation after hospital discharge. It was found that survivors struggle to access healthcare professionals and services post-discharge, influencing the drive for physical recovery. Supervised exercise programmes positively impact the perception of recovery and motivation. However, the "simple" provision of structured exercise does not address the variety of challenges experienced by ICU survivors (Herridge and Azoulay 2023).
Long-Term Advantages
Early rehabilitation has been associated with fewer hospital visits three years after discharge, shorter hospital stays, and lower healthcare costs after discharge than the late rehabilitation group (Murooka et al. 2023).
The potential of early mobilisation is not limited to counteracting the physiological consequences of critical illness in the physical recovery domain but also affects cognitive and mental function (Jackson et al. 2012). A recent review (Liu et al. 2024) summarises the current scientific evidence supporting early rehabilitation as a strategy against developing post-intensive care syndrome (PICS). The text attempts to elucidate the underlying mechanisms and analyses early rehabilitation from different perspectives: its application during ICU stay, hospital ward admission, and at home, the impact of early mobilisation on physical function, cognitive function, and the patient’s psychological dimension (social function, mood, pain, quality of life, etc.).
Three systematic reviews (Brummel et al. 2014; Denehy et al. 2013; Schweickert et al. 2009) examined the effect of early mobilisation in the ICU, focusing on physical and functional outcomes as opposed to the cognitive impacts, for which a Cochrane review (Doiron et al. 2018) could not determine any treatment effect due to the heterogeneity of interventions and small sample size. In 2023, (Patel et al. 2023) published a randomised controlled trial analysing the impact of early rehabilitation on long-term cognitive function in patients who received mechanical ventilation. In this study, early physical and occupational therapy within the first 96 hours of mechanical ventilation was associated with a substantial improvement in cognitive impairment, neuromuscular weakness, and quality of life in physical health domains (although it is not clear whether this improvement is due to the interventions performed or the needed reduction in sedative drugs). In this regard, it should be noted that early mobilisation is one of the strategies included in the recommended bundles to prevent the development of delirium in critically ill patients (Matsuura et al. 2023), with a known impact on the cognitive domain of patients. Therefore, the implementation in practice of complex multidisciplinary interventions such as early mobilisation in the acute phase of critical illness has substantial benefits on long-term disability in survivors after mechanical ventilation.
The Upcoming Future
Incorporating new/emerging technologies such as virtual reality (VR), gaming consoles, apps, and robotics may provide the necessary boost to promote rehabilitation. Examples of already undertaken measures are using apps and telehealth complementary therapies to early mobilisation (Sumner et al. 2023). Apps provide easy system accessibility and customised treatment (Anan et al. 2021; Lo et al. 2018; Thiengwittayaporn et al. 2021). VR immerses the person in a fully simulated environment with 360-degree vision and simulated active movements (Oliveira et al. 2022). The application of VR in the ICU has proven to be safe and feasible while yielding promising results in cognitive/psychological areas such as anxiety reduction, pain levels, and delirium (Jawed et al. 2021; Merliot-Gailhoustet et al. 2022; Vlake et al. 2021). In small studies, VR has proven effective in promoting early mobilisation (through complete bed or chair play) (Hemphill et al. 2021; Lai et al. 2021).
Early mobilisation has also been safely delivered in the ICU through gaming platforms like the Nintendo Wii™ virtual therapy system and Xbox Kinect Jintronix, with studies reporting high patient engagement levels and no adverse events (Abdulsatar et al. 2013; Gomes et al. 2019; Parke et al. 2020). Gaming platforms allow patients to be remotely monitored and objectively assess their progress. From the patient’s perspective, game-based exercises are attractive, easy to do, and adjusted to an appropriate difficulty level.
Other novel therapies to improve access to early mobilisation in the ICU may include interventions such as rehabilitation robotics or exoskeleton robots. Robots designed to assist in patient treatments in the ICU are primarily in the development phase or can currently only assist in manual manipulation tasks such as lifting/turning patients in bed. Exoskeletons have been proposed to facilitate out-of-bed mobilisation of ICU patients (Kosa et al. 2022; Luetz et al. 2019; Plaza et al. 2023).
What Remains to be Explored?
It has been demonstrated that early mobilisation is safe and feasible during and after ICU admission. Recent research trends have focused on exploring the optimal dosing and timing of early mobilisation administration (e.g., intensity, duration, frequency), complementary/additional interventions (e.g., clustered care, nutrition, environmental optimisation) (Mion et al. 2023; Renner et al. 2023; Singer et al. 2023), and technology/tools that can deliver early mobilisation (Ferre et al. 2021; Schrempf et al. 2023). The effects of early mobilisation on short-term outcomes (e.g., mortality, delirium, ICU length of stay, and weaning from mechanical ventilation) and long-term outcomes (e.g., PICS-related outcomes, healthcare resource utilisation, and economic and social impacts) are being examined. Research groups investigate the heterogeneous effect of early mobilisation among different ICU patient cohorts, optimising the intervention to fit patients’ background comorbidities (Narváez-Martínez and Henao-Castaño 2024). In this regard, an artificial intelligence-based learning approach has recently demonstrated the heterogeneous effect of early mobilisation in different ICU patient cohorts, suggesting the importance of an individualised and optimised resource approach (Fuest et al. 2023).
There is increasing awareness and recognition of the relationship between the physical ICU environment and patient outcomes (Huisman et al. 2012; Wenham and Pittard 2009). Patients and staff report that small, cluttered, and suboptimal physical environments can impede the best care delivery and contribute to staff injuries and poor outcomes (Tronstad et al. 2021). Recent projects have shown that it is possible to optimise ICU environments, but there is no evidence that this impacts patient outcomes (Tronstad et al. 2023). Future ICU designs must consider the recent shift in care models from sedated to awake patients and provide an environment where early rehabilitation is possible (including sufficient space to store rehabilitation equipment).
Our Insight
ICU physicians must go beyond disease resolution and adopt a culture of recovery improvement with optimal physical rehabilitation. Promoting physical rehabilitation includes timely identification of suitable candidates with established safety standards, coordination of evidence-based interventions with selective sedation pauses, mobilisation interventions, and functional outcomes at ICU discharge. Finally, patients’ experiences must be followed up and clinically evaluated to improve ICU care continually.
Despite the evolution and knowledge about the effects of early mobilisation on PICS, many gaps remain in current evidence, highlighting the need for continued thorough research, ensuring that individualised assessments and interventions are performed at the right time and continue after hospital discharge, exploring the optimisation of early mobilisation dosing, and evaluating patient outcomes while incorporating multifaceted preventive measures and predictive models. This essential work must be prioritised to ensure that ICU survivors survive and thrive in their post-ICU life.
Conflict of Interest
None.
References:
Adler J, Malone D (2012) Early mobilisation in the intensive care unit: A systematic review. Cardiopulm Phys Ther J. 23.
Bernhardt J, Langhorne PJ, Lindley RI et al. (2015) Efficacy and safety of early mobilisation within 24 h of stroke onset (AVERT): A randomised controlled trial. Lancet. 386(9988):46-55.
Bourenne J, Hraiech S, Roch A et al. (2017) Sedation and neuromuscular blocking agents in acute respiratory distress syndrome. Ann Transl Med. 5(14).
Brummel NE, Girard TD, Ely EW et al. (2014) Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: The Activity and Cognitive Therapy in ICU (ACT-ICU) trial. Intensive Care Med. 40(3):370-9.
Bussmann JB, Stam HJ (1998) Techniques for measurement and assessment of mobility in rehabilitation: A theoretical approach. Clin Rehabil. 12(6):455-64.
Chillura A, Bramanti A, Tartamella F et al. (2020) Advances in the rehabilitation of intensive care unit acquired weakness: A case report on the promising use of robotics and virtual reality coupled to physiotherapy. Medicine (Baltimore). 99(28):e20939.
Connolly B, Denehy L, Hart N et al. (2018) Physical rehabilitation core outcomes in critical illness (PRACTICE): Protocol for developing a core outcome set. Trials. 19(1):294.
De Man AME, Gunst J, Reintam Blaser A (2024) Nutrition in the intensive care unit: From the acute phase to beyond. Intensive Care Med.
Denehy L, Lanphere J, Needham DM (2017) Ten reasons why ICU patients should be mobilised early. Intensive Care Med. 43(1):86-90.
Denehy L, Skinner EH, Edbrooke L et al. (2013) Exercise rehabilitation for patients with critical illness: A randomised controlled trial with 12 months of follow-up. Crit Care. 17(4):R156.
Doiron KA, Hoffmann TC, Beller EM (2018) Early intervention (mobilisation or active exercise) for critically ill adults in the intensive care unit. Cochrane Database Syst Rev. 3.
Dong Z, Yu B, Sun Y et al. (2014) Effects of early rehabilitation therapy on patients with mechanical ventilation. World J Emerg Med. 5(1):48.
Dubb R, Nydahl P, Hermes C et al. (2016) Barriers and strategies for early mobilisation of patients in intensive care units. Ann Am Thorac Soc. 13(5):724-30.
Ferre M, Batista E, Solanas A, Martínez-Ballesté A (2021) Smart health-enhanced early mobilisation in intensive care units. Sensors (Basel). 21(16):5408.
Flaws DFJ, Laupland K, Lavana J et al. (2024) Time in ICU and post-intensive care syndrome: How long is long enough? Crit Care.
Fuest KE, Ulm B, Daum N et al. (2023) Clustering of critically ill patients using an individualised learning approach enables dose optimisation of mobilisation in the ICU. Crit Care. 27(1):1.
Goddard S, Gunn H, Kent B, Dennett R (2024) The experience of physical recovery and physical rehabilitation following hospital discharge for intensive care survivors—A qualitative systematic review. Nurs Rep. 14(1):148-63.
Gomes TT, Schujmann DS, Fu C (2019) Rehabilitation through virtual reality: Physical activity of patients admitted to the intensive care unit. Rev Bras Ter Intensiva. 31(4).
Greening NJ, Williams JEA, Hussain SF et al. (2014) An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: Randomised controlled trial. BMJ. 349(jul08 5):g4315-g4315.
Harvey MA, Davidson JE (2016) Postintensive care syndrome: Right care, right now...and later. Crit Care Med. 44(2):381-5.
Hemphill S, Nguyen A, Kwong J et al. (2021) Virtual reality facilitates engagement in physical therapy in the pediatric CVICU. Pediatr Phys Ther. 33(1):E7-9.
Herridge MS, Azoulay É (2023) Outcomes after critical illness. N Engl J Med. 388(10):913-24.
Hodgson CL, Berney S, Harrold M et al. (2012) Clinical review: Early patient mobilisation in the ICU. Crit Care. 17(1):207.
Hodgson CL, Schaller SJ, Nydahl P et al. (2021) Ten strategies to optimise early mobilisation and rehabilitation in intensive care. Crit Care. 25(1).
Hodgson CL, Bailey M, Bellomo R et al. (2022) Early active mobilisation during mechanical ventilation in the ICU. N Engl J Med. 387(19):1747-58.
Huebner L, Schroeder I, Kraft E et al. (2022) Frühmobilisation auf der Intensivstation – Sind robotergestützte Systeme die Zukunft? Anaesthesiologie. 71(10):795-800.
Huisman ERCM, Morales E, Van Hoof J, Kort HSM (2012) Healing environment: A review of the impact of physical environmental factors on users. Build Environ. 58:70-80.
Jackson JC, Ely EW, Morey MC et al. (2012) Cognitive and physical rehabilitation of intensive care unit survivors: Results of the RETURN randomised controlled pilot investigation. Crit Care Med. 40(4):1088-97.
Jawed YT, Golovyan D, Lopez D et al. (2021) Feasibility of a virtual reality intervention in the intensive care unit. Heart Lung. 50(6):748-53.
Kho ME, Berney S, Connolly B (2023) Physical rehabilitation in the intensive care unit: Past, present, and future. Intensive Care Med. 49(7):864-7.
Kho ME, Connolly B (2023) From strict bedrest to early mobilisation. Crit Care Clin. 39(3):479-502.
Kirkham JJ, Williamson P (2022) Core outcome sets in medical research. BMJ Med. 1(1):e000284.
Kosa G, Morozov O, Lehmann A et al. (2022) Robots and intelligent medical devices in the intensive care unit: Vision, state of the art and economic analysis.
Lai B, Powell M, Clement AG et al. (2021) Examining the feasibility of early mobilisation with virtual reality gaming using head-mounted display and adaptive software with adolescents in the pediatric intensive care unit: Case report. JMIR Rehabil Assist Technol. 8(2):e28210.
Lang JK, Paykel MS, Haines KJ, Hodgson CL (2020) Clinical practice guidelines for early mobilisation in the ICU: A systematic review. Crit Care Med. 48(11):E1121-E1128.
Latronico N, Herridge M, Hopkins RO et al. (2017) The ICM research agenda on intensive care unit-acquired weakness. Intensive Care Med. 43(9):1270-1281.
Lau VI, Johnson JA, Bagshaw SM et al. (2022) Health-related quality-of-life and health-utility reporting in critical care. World J Crit Care Med. 11(4):236-245.
Lindholz M, Schellenberg CM, Grunow JJ et al. (2022) Mobilisation of critically ill patients receiving norepinephrine: A retrospective cohort study. Crit Care. 26(1).
Liu K, Shibata J, Fukuchi K et al. (2022) Optimal timing of introducing mobilisation therapy for ICU patients with sepsis. J Intensive Care. 10(1).
Liu K, Tronstad O, Flaws D et al. (2024) From bedside to recovery: Exercise therapy for prevention of post-intensive care syndrome. J Intensive Care. 12(1).
Lorenz M, Fuest K, Ulm B et al. (2023) The optimal dose of mobilisation therapy in the ICU: A prospective cohort study. J Intensive Care. 11(1).
Luetz A, Grunow JJ, Mörgeli R et al. (2019) Innovative ICU solutions to prevent and reduce delirium and post-intensive care unit syndrome. Semin Respir Crit Care Med. 40(5):673-686.
Matsuura Y, Ohno Y, Toyoshima M, Ueno T (2023) Effects of non-pharmacologic prevention on delirium in critically ill patients: A network meta-analysis. Nurs Crit Care. 28(5):727-737.
Merliot-Gailhoustet L, Raimbert C, Garnier O et al. (2022) Discomfort improvement for critically ill patients using electronic relaxation devices: Results of the cross-over randomised controlled trial E-CHOISIR (Electronic-CHOIce of a System for Intensive care Relaxation). Crit Care. 26(1):263.
Mion LC, Tan A, Brockman A et al. (2023) An exploration of critical care professionals' strategies to enhance daily implementation of the Assess, Prevent, and Manage Pain; Both Spontaneous Awakening and Breathing Trials; Choice of Analgesia and Sedation; Delirium Assess, Prevent, and Manage; Early Mobility and Exercise; and Family Engagement and Empowerment: A group concept mapping study. Crit Care Explor. 5(3):e0872.
Moraes FDS, Marengo LL, Moura MDG et al. (2022) ABCDE and ABCDEF care bundles: A systematic review of the implementation process in intensive care units. Medicine (Baltimore). 101(25):E29499.
Murooka Y, Sasabuchi Y, Takazawa T et al. (2023) Long-term prognosis following early rehabilitation in the ICU: A retrospective cohort study. Crit Care Med. 51(8):1054-1063.
Nadeau SE, Wu SS, Dobkin BH et al. (2013) Effects of task-specific and impairment-based training compared with usual care on functional walking ability after inpatient stroke rehabilitation. Neurorehabil Neural Repair. 27(4):370-380.
Narváez-Martínez MA, Henao-Castaño ÁM (2024) Severity classification and influencing variables of the post-intensive care syndrome. Enferm Intensiva. 35(2):89-96.
Oliveira I, Afonso A, Oliveira E et al. (2022) Design principles for cognitive and physical rehabilitation of ICU patients using Virtual Reality (VR). 2022 IEEE 10th International Conference on Serious Games and Applications for Health (SeGAH). 1-7.
Parke S, Hough CL, Bunnell AE (2020) The feasibility and acceptability of virtual therapy environments for early ICU mobilisation. PM R. 12(12):1214-1221.
Patel BK, Wolfe KS, Patel SB et al. (2023) Effect of early mobilisation on long-term cognitive impairment in critical illness in the USA: A randomised controlled trial. Lancet Respir Med. 11(6):563-572.
Paton M, Chan S, Serpa Neto A et al. (2024) Association of active mobilisation variables with adverse events and mortality in patients requiring mechanical ventilation in the intensive care unit: A systematic review and meta-analysis. Lancet Respir Med. 12(5):386-398.
Paton M, Chan S, Tipping CJ et al. (2023) The effect of mobilisation at 6 months after critical illness—Meta-analysis. NEJM Evid. 2(2).
Paton M, Lane R, Paul E et al. (2021) Mobilisation during critical illness: A higher level of mobilisation improves health status at 6 months, a secondary analysis of a prospective cohort study. Crit Care Med. 49(9):E860-E869.
Peper KK, Aasmann A, Jensen ER, Haddadin S (2023) Real-time-capable muscle force estimation for monitoring robotic rehabilitation therapy in the intensive care unit. 2023 45th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC). 1-6.
Plaza A, Hernandez M, Puyuelo G et al. (2023) Wearable rehabilitation exoskeletons of the lower limb: Analysis of versatility and adaptability. Disabil Rehabil Assist Technol. 18(4):392-406.
Renner C, Jeitziner MM, Albert M et al. (2023) Guideline on multimodal rehabilitation for patients with post-intensive care syndrome. Crit Care. 27(1).
Schaller SJ, Anstey M, Blobner M et al. (2016) Early, goal-directed mobilisation in the surgical intensive care unit: A randomised controlled trial. Lancet. 388(10052):1377-1388.
Scheffenbichler FT, Teja B, Wongtangman K et al. (2021) Effects of the level and duration of mobilisation therapy in the surgical ICU on the loss of the ability to live independently: An international prospective cohort study. Crit Care Med. 49(3):e247-e257.
Scheunemann LP, White JS, Prinjha S et al. (2020) Post–intensive care unit care: A qualitative analysis of patient priorities and implications for redesign. Ann Am Thorac Soc. 17(2):221-228.
Schrempf MC, Zanker J, Arndt TT et al. (2023) Immersive virtual reality fitness games to improve recovery after colorectal surgery: A randomised single-blind controlled pilot trial. Games Health J. 12(6):450-458.
Schujmann DS, Teixeira Gomes T, Lunardi AC et al. (2020) Impact of a progressive mobility program on the functional status, respiratory, and muscular systems of ICU patients: A randomised and controlled trial. Crit Care Med. 48(4):491-497.
Schweickert WD, Pohlman MC, Pohlman AS et al. (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomised controlled trial. Lancet. 373(9678):1874-1882.
Settembre N, Maurice P, Paysant J et al. (2020) The use of exoskeletons to help with prone positioning in the intensive care unit during COVID-19. Ann Phys Rehabil Med. 63(4):379-382.
Singer P, Blaser AR, Berger MM et al. (2023) ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin Nutr. 42(9):1671-1689.
Stevens RD, Marshall SA, Cornblath DR et al. (2009) A framework for diagnosing and classifying intensive care unit-acquired weakness. Crit Care Med. 37(Suppl 10).
Sumner J, Lim HW, Chong LS et al. (2023) Artificial intelligence in physical rehabilitation: A systematic review. Artif Intell Med. 146:102693.
The TEAM Study Investigators and the ANZICS Clinical Trials Group (2022) Early active mobilisation during mechanical ventilation in the ICU. N Engl J Med. 387(19):1747-1758.
Tronstad O, Flaws D, Lye I et al. (2021) The intensive care unit environment from the perspective of medical, allied health and nursing clinicians: A qualitative study to inform design of the 'ideal' bedspace. Aust Crit Care. 34(1):15-22.
Tronstad O, Flaws D, Patterson S et al. (2023) Creating the ICU of the future: Patient-centred design to optimise recovery. Crit Care. 27(1):402.
Unoki T, Hayashida K, Kawai Y et al. (2023) Japanese Clinical Practice Guidelines for Rehabilitation in Critically Ill Patients 2023 (J-ReCIP 2023). J Intensive Care. 11(1):47.
Van Willigen Z, Ostler C, Thackray D, Cusack R (2020) Patient and family experience of physical rehabilitation on the intensive care unit: A qualitative exploration. Physiotherapy. 109:102-110.
Vanhorebeek I, Latronico N, Van den Berghe G (2020) ICU-acquired weakness. Intensive Care Med. 46(4):637-653.
Vlake JH, Van Bommel J, Wils EJ et al. (2021) Virtual Reality to Improve Sequelae of the Postintensive Care Syndrome: A Multicenter, Randomized Controlled Feasibility Study. Crit Care Explor. 3(9):e0538.
Vollenweider R, Manettas AI, Häni N et al. (2022) Passive motion of the lower extremities in sedated and ventilated patients in the ICU – a systematic review of early effects and replicability of Interventions. PLoS One. 17(5 May):e0267255.
Waldauf P, Jiroutková K, Krajčová A et al. (2020) Effects of Rehabilitation Interventions on Clinical Outcomes in Critically Ill Patients: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit Care Med. 48(7):1055-1065.
Walsh TS, Salisbury LG, Merriweather JL et al. (2015) Increased Hospital-Based Physical Rehabilitation and Information Provision After Intensive Care Unit Discharge. JAMA Intern Med. 175(6):901.
Wang YT, Lang JK, Haines KJ et al. (2022) Physical Rehabilitation in the ICU: A Systematic Review and Meta-Analysis. Crit Care Med. 50(3):375-388.
Watanabe S, Morita Y, Suzuki S et al. (2021) Effects of the Intensity and Activity Time of Early Rehabilitation on Activities of Daily Living Dependence in Mechanically Ventilated Patients. Prog Rehabil Med. 6(0):20210054.
Wenham T, Pittard A (2009) Intensive care unit environment. Contin Educ Anaesth Crit Care Pain. 9(6):178-183.
World Health Organization (2023) Rehabilitation. Available at https://www.who.int/news-room/fact-sheets/detail/rehabilitation
Wright SE, Thomas K, Watson G et al. (2018) Intensive versus standard physical rehabilitation therapy in the critically ill (EPICC): A multicentre, parallel group, randomised controlled trial. Thorax. 73(3):213-221.
Yang R, Zheng Q, Zuo D et al. (2021) Safety Assessment Criteria for Early Active Mobilisation in Mechanically Ventilated ICU Subjects. Respir Care. 66(2):307-315.
Yang T, Li Z, Jiang L, Wang Y, Xi X (2018) Risk factors for intensive care unit-acquired weakness: A systematic review and meta-analysis. Acta Neurol Scand. 138(2):104-114.
Yang Z, Wang X, Wang F et al. (2022) A systematic review and meta-analysis of risk factors for intensive care unit acquired weakness. Medicine (Baltimore). 101(43):e31405.
Yébenes JC, Bordeje-Laguna ML, Lopez-Delgado JC et al. (2024) Smartfeeding: A Dynamic Strategy to Increase Nutritional Efficiency in Critically Ill Patients—Positioning Document of the Metabolism and Nutrition Working Group and the Early Mobilization Working Group of the Catalan Society of Intensive and Critical Care Medicine (SOCMiC). Nutrients. 16(8):1157.
Zaga CJ, Wallace S, Freeman-Sanderson A (2024) Letter to the editor in response to the Japanese clinical practice guidelines for rehabilitation in critically ill patients 2023 (J-ReCIP 2023). J Intensive Care. 12(1):25









