ICU Management & Practice, Volume 22 - Issue 1, 2022
Introduction
For decades, the focus of patient management in the Intensive Care Unit (ICU) has been to perform a large number of interventions in critically ill patients, many of which are based on clinical judgment and the pathophysiology of diseases. However, evidence for such practices many times does not support them. We present 10 common clinical situations in which doing more could be associated with a higher risk of worse outcomes.
1. Fluid Overload
Intravenous (IV) fluid therapy is the mainstay treatment for patients with hypovolaemia, commonly due to blood loss or dehydration. However, it has been shown that <50% patients in the ICU can be categorised as responders to IV fluids. Unwarranted IV fluid prescription can be unfavourable since fluid overload leads to endothelial damage with direct involvement of the glycocalyx, increased vascular permeability to the extracellular space, increased pressure in encapsulated organs, and multisystem oedema.
Adverse events most frequently related to volume overload are acute kidney injury (AKI), prolonged hospital stay, pulmonary oedema, effusions, increased days on invasive mechanical ventilation (IMV) and higher mortality (Malbrain 2018; Pérez-Nieto 2021).
It is common for patients with AKI in the ICU to be treated aggressively with IV fluids. Nonetheless, congestive renal failure related to irrational fluid therapy is associated with worse outcomes as shown in multicentre studies such as REVERSE-AKI 2021 and FINNAKITRIAL, in which restrictive fluid therapy strategies were associated with less adverse effects including overall cumulative fluid balance and mortality.
In septic shock, the Surviving Sepsis Campaign recommendations published in 2021 recommend aggressive IV fluid therapy with crystalloids at a dose of 30 ml/kg. However, evidence supporting this recommendation is weak and increasingly questioned since multiple cohort studies have shown that only 3% of patients with septic shock will be fluid responsive within eight hours of admission and will no longer benefit from fluid therapy (Pittard 2017; Cordemans 2012; Flori 2011). Furthermore, a positive fluid balance of more than 2 L is associated with increased mortality.
The role of hidden fluid must also be taken into consideration, as it accounts for about a third of the cumulative
water balance involving fluid from drug vials, intravenous lines, enteral nutrition, and blood products, making the intention of a benefit a cause of harm (Branan 2020). IV fluid therapy in the critically ill patient must be justified millilitre by millilitre and overload must be avoided at all costs.
2. Oversedation
Sedatives are commonly used in the ICU. Sedation is indicated in patients with moderate to severe acute respiratory distress syndrome (ARDS), patients with intracranial hypertension (ICH) and other scenarios. The drugs of choice are propofol and dexmedetomidine. However, a large proportion of patients do not require sedation and could be managed with adequate analgesia only and, in case of agitation, anxiolytics or antipsychotics (Park 2019).
Unnecessary sedation is harmful for critically ill patients. A recently published re-analysis of the NON-SEDA study showed that patients who remained sedated for agitation or respiratory failure had worse outcomes, including more IMV and ICU days, as well as a higher incidence of delirium, despite no impact on mortality (Nedergaard 2022). Prolonged sedation limits early rehabilitation with active mobilisation. Benzodiazepines as sedative agents are associated with worse outcomes and are not recommended as first choices (Park 2019). In patients with ARDS, daily interruption of sedation has been shown to be associated with decreased days of IMV, hospital stay, and mortality (Kress 2000). Combining this strategy with a daily spontaneous ventilation test can lead to better results (Girard 2008).
3. Irrational Use of Antibiotics
Sepsis is one of the most frequent diagnoses in the ICU. Early treatment with antibiotics (<1 h) has been associated with better outcomes (Kollef 2021). Nevertheless, there are patients without confirmed or suspected infections who do not require antibiotics. Unjustified antibiotic prescription contributes to antimicrobial resistance, which is already a problem in most hospitals with high incidence of infections by multidrug resistant pathogens. Adverse effects that can occur when using unnecessary antibiotics include mild to severe gastrointestinal disorders (i.e., Clostridioides difficile infection), arrhythmias (azithromycin), seizures (carbapenems), etc. With suspected infection, cultures should always be requested, and therapy adjusted, as antimicrobial stewardship is safe and associated with fewer complications (Ilges 2021) and lower mortality.
During the COVID-19 pandemic, inappropriate antibiotic treatment has been at its peak. Azithromycin and other macrolides, nitazoxanide, ivermectin, cephalosporins, and other drugs have been indicated without evidence of benefit (RECOVERY trial 2020-2021). The overall impact of this therapeutic misconduct remains to be characterised.
4. Prophylaxis of Gastrointestinal Ulcers
Proton pump inhibitors (PPI) and histamine-2-receptor antagonists (H2A) are commonly used in critically ill patients to prevent gastrointestinal ulcers by decreasing acid production. Nevertheless, this acid is a barrier to external pathogens, reason why suppressing their secretion could promote intestinal and lung infections. PPIs may also cause alterations in leukocyte function phagocytosis, and acidification of the lytic phagolysosome (Buendgens 2014; McDonald 2015).
There are many questions regarding whether there is benefit from their routine use or not, especially in the absence of clear indication such as upper gastrointestinal bleeding. Studies differ in proving the benefit in groups using these interventions. On the other hand, adverse events can be increased. For instance, mechanical ventilator-associated pneumonia (VAP), Clostridioides difficile infection (Trifan 2017), increased hospital stay, and no reductions in mortality (Alhazzani 2017; Marker 2018). Enteral nutrition itself may be associated with decreased risk of gastrointestinal ulcers (Huang 2018).
5. Inappropriate Blood Transfusions
Transfusion of blood products in critically ill patients has precise indications, such as haemorrhagic shock, severe anaemia, or coagulopathy. Unnecessary administration of blood products is associated with complications including increased length of hospital stay, transfusion related acute lung injury (TRALI), transfusion associated circulatory overload (TACO), increased costs, and higher mortality (Fung 2019).
The lack of knowledge of standardised blood product transfusion protocols results in the irrational use in the ICU (Spahn 2019). Currently, restrictive transfusion therapy is associated with better outcomes, and it may be better not to transfuse when haemoglobin levels are between 7-8 g/dl without active or massive bleeding (Alexander 2021). Guiding the amount and type of transfusions by viscoelastic tests has also not been shown to be better when compared to conventional coagulation tests (ITACTIC trial 2020).
6. Abuse and Misuse of Laboratory Tests
Blood tests for critically ill patients in the ICU have become routine rather than being based on diagnostic workups. Blood sampling should only be justified on the principle of objective intervention (Angus 2014). The usual indication of ordering daily blood samples from patients represents the unnecessary and unjustifiable retirement of 40-70 ml of blood every 24h (Ñamendys 2019). Consequently, a decrease in haemoglobin of about
1-1.2 g per day has been demonstrated (Fung 2019), leading to iatrogenic anaemia that may even require transfusion of blood products (Smoller 1989). Prospective trials should aim to reduce the volume of sample collected (paediatric phlebotomy tubes, reduced volumes of syringes, etc.).
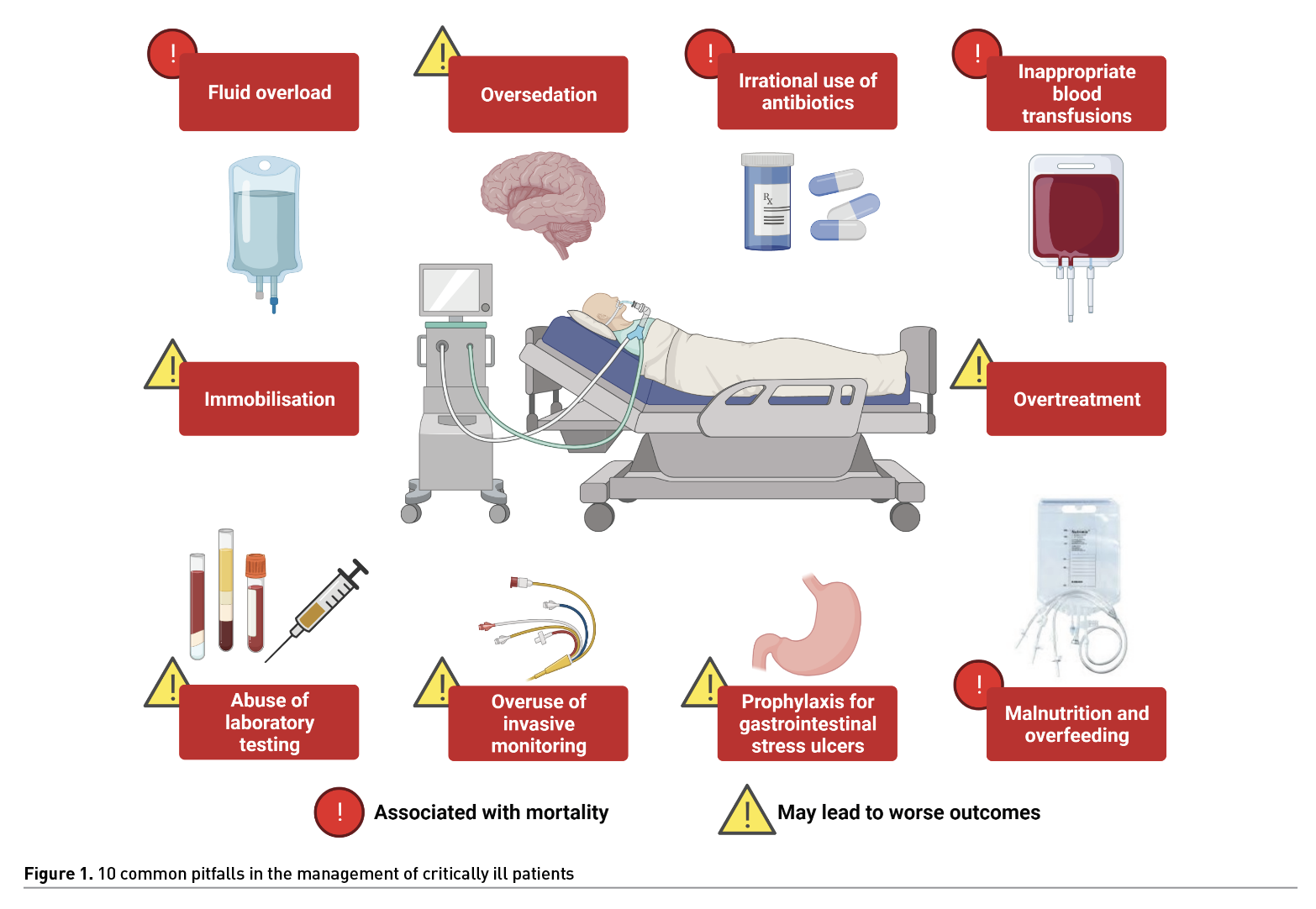
7. Invasive Monitoring
Pulmonary artery catheterisation - Swan-Ganz catheterisation - was popularised in the 1970s to perform invasive monitoring in the ICU by providing the estimated value of cardiac output through thermodilution and measurement of right heart chamber pressures as well as pulmonary circulation. By the end of the last century, a high rate of serious complications associated with this procedure were reported. Several clinical trials failed to demonstrate the benefit of this technique for critically ill patients, reason why it began to be discontinued (Marik 2013). As a risky procedure that requires trained medical and nursing staff to perform the measurements properly, with greater time and resources demands, this technique has now been abandoned in most ICUs. The debate of its usefulness in patients undergoing cardiac surgery is still ongoing (Rozental 2021).
Transpulmonary thermodilution (TPT) is an invasive tool that requires the placement of a central venous line (jugular or subclavian) and an arterial line (usually femoral, brachial or radial), that provides information on the macrohaemodynamic (cardiac output, systemic vascular resistances, volume statues, etc.) and respiratory status of the patient (extravascular lung water and pulmonary vascular permeability index). It is used in some ICUs or operating rooms for the management of complex patients (Monnet 2017). However, using it to guide haemodynamic management has not been shown to reduce mortality and only improves perfusion in hypotensive patients (Li 2021). There have been reports of thrombosis and other vascular complications due to the placement of arterial lines, in addition to the complications inherent to central venous catheterisation. More studies are required to elucidate the usefulness of invasive devices for haemodynamic monitoring in the ICU.
8. Malnutrition and Overfeeding
Patients with circulatory shock may benefit from short periods of fasting to avoid intestinal ischaemia while their macro- and micro-haemodynamic status improves. Despite this, prolonged fasting and hospital malnutrition have been shown to be associated with poorer outcomes and higher mortality (Galindo-Martín 2018).
It is currently recommended to start with an enteral nutrition (EN) tolerance test at a trophic dose within 48 h of admission, aiming to cover 100% calorie requirement (20-30 kcal/kg/day) within 3-7 days of the onset of critical illness (ESPEN 2021). Starting EN with a full-dose calorie intake has not been shown to reduce mortality but can reduce the incidence of adverse events including gastrointestinal intolerance, episodes of hyperglycaemia, and increased insulin requirement (EDEN randomised trial 2012; EAT-ICU trial 2017). Low protein intake is associated with higher rates of infection and mortality in critically ill patients. Thus, it should be included in the nutritional intake (0.8-1.2 g Prot/kg/day). Intakes>1.2 g Prot/kg/day have not been shown to improve outcomes (Lee 2021; Hartl 2022). The cost of nutritional therapy, which may include calorie, protein, fat, or trace element supplements, must also be taken into account.
9. Overtreatment
Overtreatment includes performing interventions that are not desired by the patient and/or do not generate any benefit for the patient. Critically ill patients with chronic terminal illnesses or severe acute pathologies complicated by irreversible organ failure are often subjected to supportive therapies such as sedation, neuromuscular blockade, fluid therapy, vasopressors, inotropics, blood products, nutrition, antibiotics, and other drugs, which will not increase their chance of survival and will only increase days of hospital stay and inappropriate use of resources (lab and imaging studies, drugs, surgeries, etc.), including ICU admission itself (Druml 2019).
The following measures have been proposed for the prevention and recognition of overtreatment in the ICU: 1) Frequent evaluation of therapeutic goals within the medical team in charge, always taking into account the wishes of the patient and their family; 2) high quality multidisciplinary management; 3) minimise treatment costs and expenses; 4) strengthen multidisciplinary cooperation through education and training; and 5) promoting social discourse on overtreatment (Michalsen 2021). Humanisation and palliative care programmes should be implemented with the aim of relieving or reducing the patient’s pain and suffering, without resorting to futile therapies.
10. Immobilisation
Most critically ill patients remain immobilised, mainly when they are in IMV, shock or with severe neurological conditions. Prolonged immobilisation has serious consequences, such as weakness (polyneuropathy or myopathy), risk of venous embolism, pressure ulcers, etc. There is a widespread fear of frequent mobilisation, as it is commonly believed that a patient requiring vasopressor, mechanical ventilation, continuous renal replacement therapy or even ECMO should not be mobilised.
Rehabilitation should start in the ICU. The benefits of early mobilisation include improved muscle strength, increased patient independence, minimising the complications and risks described above, and favours domiciliary adaptation (Zhang 2019). It should be performed by trained physical therapy specialists and initiated when the patient is at minimal or no significant risk of complications, always following safety parameters, for which it is necessary to monitor vital signs, cardiovascular, neurological and respiratory status (Martinez-Camacho 2021).
Conclusion
The conduct of “doing more” in the management of critically ill patient does not always generate benefits and may carry risks. In the ICU, we must justify our medical decisions based on the best available evidence and only apply further therapeutic measures when improved outcomes have been demonstrated.
Conflict of Interest
None.
References:
Agastya G, West BC, Callahan JM (2000) El omeprazolinhibe la fagocitosis y la acidificación de los fagolisosomas de los neutrófiloshumanosnormales in vitro. Inmunofarmaco Inmunotoxicol. 22 :357–372.
Alhazzani W, Alshamsi F, Belley-Cote E et al. (2018) Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med. 44(1):1-11.
Allingstrup MJ, Kondrup J, Wiis J et al. (2017) Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 43(11):1637-1647.
Angus DC, Deutschman CS, Hall JB et al. (2014) Choosing Wisely1 in Critical Care. Crit Care Med. 42:2437–2438.
Baksaas-Aasen K, Gall LS, Stensballe J et al. (2021) Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): a randomized, controlled trial. Intensive Care Med. 47(1):49-59.
Branan T, Smith SE, Newsome AS et al. (2020) Association of hidden fluid administration with development of fluid overload reveals opportunities for targeted fluid minimization. SAGE Open Med. 8:2050312120979464.
Buendgens L, Bruensing J, Matthes M et al. (2014) Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile-associated diarrhea. J Crit Care. 29(4):696.e11-5.
Carson JL, Guyatt G, Heddle NM et al. (2016) Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 316(19):2025–2035.
Cordemans C, De Laet I, Van Regenmortel N et al. (2012) Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care. 5;2(Suppl 1 Diagnosis and management of intra-abdominal hyperten):S1.
Corwin HL, Parsonnet KC, Gettinger A (1995) RBC transfusion in the ICU. Is there a reason? Chest. 108(3):767-771.
Cuerda C, Pironi L, Arends J et al. (2021) Home Artificial Nutrition & Chronic Intestinal Failure Special Interest Group of ESPEN. ESPEN practical guideline: Clinical nutrition in chronic intestinal failure. Clin Nutr. 40(9):5196-5220.
Druml W, Druml C (2019) Overtreatment in intensive care medicine. Med KlinIntensiv med Notfmed. 114(3):194-201.
Flori HR, Church G, Liu KD et al. (2011) Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. 854142.
Foulke GE, Harlow DJ (1989) Effective measures for reducing blood loss from diagnostic laboratory tests in intensive care unit patients. Crit Care Med. 17(11):1143-5.
Fung CM, Hyzy RC (2019)Deadoption of low-value practices in the ICU. CurrOpin Crit Care. 25(5):517-522.
Galindo-Martin CA, Monares-Zepeda E, Pérez-Nieto OR (2018). Enteral nutrition (early) and hemodynamic status in the critically ill patient: What should the nutritional support clinician know?Journal of Clinical Nutrition and Metabolism. 1(1), 53-63.
Girard TD, Kress JP, Fuchs BD et al. (2008) Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 371(9607):126-34.
Hartl WH, Kopper P, Bender A et al. (2022) Protein intake and outcome of critically ill patients: analysis of a large international database using piece-wise exponential additive mixed models. Crit Care 26, 7.
Heffner JE (2000) A Wake-Up Call in the Intensive Care Unit. New England Journal of Medicine. 342(20):1520–1522.
Huang HB, Jiang W, Wang CY et al. (2018). Stress ulcer prophylaxis in intensive care unit patients receiving enteral nutrition: a systematic review and meta-analysis. Critical care (London, England). 22(1):20.
Ilges D, Ritchie DJ, Krekel T et al. (2021Assessment of Antibiotic De-escalation by Spectrum Score in Patients With Nosocomial Pneumonia: A Single-Center, Retrospective Cohort Study. Open Forum Infect Dis. 17;8(11):ofab508.
Kollef MH, Shorr AF, Bassetti M et al. (2021) Timing of antibiotic therapy in the ICU. Crit Care. 25, 360.
Kress JP, Pohlman AS, O'Connor MF, Hall JB (2000) Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 342(20):1471-7.
Lee ZY, Yap CSL, Hasan MS et al. (2021) The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 25(1):260.
Li C, Wang S, Wang H et al. (2021) The effects of hemodynamic monitoring using the PiCCO system on critically ill patients. American journal of translational research. 13(9): 10578–10585.
Marik PE (2013) Obituary: pulmonary artery catheter 1970 to 2013. Ann Intensive Care. 3(1):38.
Marker S, Krag M, Perner A et al. (2019) Pantoprazole in ICU patients at risk for gastrointestinal bleeding-1-year mortality in the SUP-ICU trial. Acta Anaesthesiol Scand. 63(9):1184-1190.
Martínez CM, Jones BRA, Gómez GA et al. Movilizacióntempranaen la Unidad de CuidadosIntensivos. Med Crit. 35(2):89-95.
McDonald EG, Milligan J, Frenette C, Lee TC (2015) Continuous Proton Pump Inhibitor Therapy and the Associated Risk of Recurrent Clostridium difficile Infection. JAMA Intern Med. 175:784–791.
Michalsen A, Neitzke G, Dutzmann J et al. (2021) Overtreatment in intensive care medicine-recognition, designation, and avoidance: Position paper of the Ethics Section of the DIVI and the Ethics section of the DGIIN]. Med KlinIntensiv med Notf med. 116(4):281-294.
Monnet X, Teboul JL (2017) Transpulmonary thermodilution: advantages and limits. Crit Care. 21(1):147.
Muñoz-Martínez T (2012) Interrupcióndiaria de la sedación; ¿siempre es un indicador de calidad? MedicinaIntensiva. 36(4), 288–293.
Ñamendys-Silva SA (2019) Cuidado de Alto Valoren Medicina Crítica. Med Crit. 33(2):91-97.
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Rice TW, Wheeler AP et al. (2012) Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 307(8):795-803. doi: 10.1001/jama.2012.137.
Nedergaard HK, Korkmaz S, Olsen HT et al. (2021) Failure of non-sedation strategy in critically ill, mechanically ventilated patients - a retrospective, post-hoc analysis of the NONSEDA trial. J Crit Care. 68:66-71.
Nisula, S, Kaukonen KM, Vaara ST et al. (2013) Incidencia, factores de riesgo y mortalidad a 90 días de pacientes con insuficiencia renal agudaenunidades de cuidadosintensivosfinlandeses: elestudio FINNAKI. Intensive Care Med.39, 420–428.
Park SY, Lee HB (2019) Prevention and management of delirium in critically ill adult patients in the intensive care unit: a review based on the 2018 PADIS guidelines. Acute Crit Care. 34(2):117-125.
Perez Nieto OR, Wong A, Lopez Fermin J, Malbrain M (2021) Aiming for zero fluid accumulation: First, do no harm. Anaesthesiol Intensive Ther. 53(2):162-178.
Pittard MG, Huang SJ, McLean AS, Orde SR (2017) Association of positive fluid balance and mortality in sepsis and septic shock in an Australian cohort. Anaesth Intensive Care. 45(6):737-743.
Randomised Evalutation of COVID-19 Therapy. Avaiable atwww.recoverytrial.net/results
Rozental O, Thalappillil R, White RS, Tam CW (2021) To Swan or Not to Swan: Indications, Alternatives, and Future Directions. J CardiothoracVascAnesth. 35(2):600-615.
Silversides JA, Perner A, Malbrain MLNG (2019) Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med. 45(10):1440-1442.
Smoller BR, Kruskall MS (1986) Phlebotomy for diagnostic laboratory tests in adults. Pattern of use and effect on transfusion requirements. N Engl J Med. 314(19):1233-5.
Spahn DR, Bouillon B, Cerny V et al. (2019) The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care 23, 98.
Thorens J, Froehlich F, Schwizer W et al. (1996) Sobrecrecimientobacterianoduranteeltratamiento con omeprazolencomparación con cimetidina: un estudioprospectivoaleatorizado doble ciego. Intestino. 39:54–59
Trifan A, Stanciu C, Girleanu I, Stoica OC et al. (2017) Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol. 23(35):6500-6515.
Vaara ST, Ostermann M, Bitker L et al. (2021) Restrictive fluid management versus usual care in acute kidney injury (REVERSE-AKI): a pilot randomized controlled feasibility trial. Intensive Care Med.47(6):665-673.
Vincent JL (2005) Give your patient a fast hug (at least) once a day. Crit Care Med. 33(6):1225-9.
Vlaar APJ, Dionne JC, de Bruin S et al. (2021) Transfusion strategies in bleeding critically ill adults: a clinical practice guideline from the European Society of Intensive Care Medicine. Intensive Care Med. 47(12):1368-1392.
Xiaojuan C (2015) Daily sedation interruption in critically ill patients on mechanical ventilation. Am J Nurs.115(5):21.
Zedtwitz-Liebenstein K, Wenisch C, PatrutaS et a. (2002) Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit Care Med. 30(5):1118-22.
Zhang L, Hu W, Cai Z et al. (2019) Early mobilization of critically ill patients in the intensive care unit: A systematic review and meta-analysis. PLoS One. 14(10):e0223185.












