ICU Management & Practice, Volume 23 - Issue 2, 2023
During artificial organ support, kidney injury is multifactorial and related to the high severity of patients treated with extracorporeal membrane oxygenation (ECMO). The successful delivery of continuous renal replacement therapy (CRRT) during ECMO requires a clear prescription of the target solute clearance and fluid removal rate based on the cumulative fluid balance and physiological variables. The role of CRRT and the optimal time, modality, and dose still need clarification.
Introduction
Extracorporeal membrane oxygenation (ECMO) is a widely recognised lifesaving support strategy for the management of patients with severe heart or/and lung failure of various aetiologies. ECMO finds its place when other conventional therapeutic strategies fail (Eckman et al. 2019). Providing support of the lung and/or the heart function, ECMO is a heart and/or lung bridge therapy for resting or replacing organ functioning.
According to the Extracorporeal Life Support Organisation (ELSO) Registry, ECMO patients are at high risk of Acute Kidney injury (AKI); the incidence ranges from 20% to as high as 85% in specific populations (i.e., neonates with congenital diaphragmatic hernia or congenital heart disease). This high variation in reported incidences is based on differences in patients’ characteristics, clinical settings and the use of diverse methods to outline AKI (Ostermann and Lumlertgul 2021).
During artificial organ support, the kidney injury is multifactorial and particularly related to the high severity of patients treated with ECMO and the lung-kidney (Gu et al. 2021). Indeed, additional circuit-related risk factors for AKI during ECMO system are represented by non-pulsatile blood flow, blood exposure to the artificial surfaces of ECMO circuit, red cell stress, haemolysis, bleeding, coagulopathy, limb ischaemia, and infection. Even more, patients requiring ECMO with high blood flows are particularly prone to develop fluid overload (FO) that is associated with prolonged use of ECMO and AKI (Patil and Salunke 2020).
Fluid balance (FB) is a fundamental aspect of critical care practice and the association between FO and worse outcome is widely recognised in intensive care (Samoni et al. 2016). For this reason, the assessment of the hydration status, for a tailored FB, represents a central and critical aspect of patients’ management (Kalantari et al. 2013). Although volume resuscitation strategy is indispensable for the intravascular volume preservation and organ perfusion, salt and water intake excess can lead to tissue oedema, increasing venous pressure with consequent altered renal blood flow, contributing to ongoing organ dysfunction (Ostermann et al. 2014). Nevertheless, FO plays a well-known negative impact on lung function and recovery. A tight FB evaluation may allow the administration of adequate nutrition and blood production avoiding further fluid accumulation with less diuretic intake. Consequently, conservative fluid administration and strategy to prevent or treat FO are essential in critically ill patients, likewise, in ECMO patients (Silversides et al. 2017).
Major indications for renal replacement therapy (RRT) during ECMO are represented by prevention of FO (16%), treatment of FO (43%), AKI (35%) and electrolyte imbalance (4%) (Fleming et al. 2012). RRT during ECMO is usually provided as a continuous modality (CRRT) because of the haemodynamic instability characteristic of such patients. CRRT allows a more constant and reliable fluid elimination and electrolyte adjustment over a longer period of time. ECMO and CRRT can be combined in two main modalities; parallel approach vs integrated application (Martins-Costa et al. 2022). The choice of the modalities is influenced, generally, by local experience and technical availability. Both techniques present advantages and disadvantages and it is vital to know the drawback and the potential of such technologies before CRRT initiation (Ostermann et al. 2018).
Central aspect of effective delivery of CRRT during ECMO is represented by a clear prescription of the target solute clearance and fluid removal rate with regular reassessment and re-adjustment of the prescription based on the changing needs of the patient (Tandukar and Palesvsky 2019). Finally, while the deleterious impact of AKI and FO on outcomes for ECMO patients is clear, critical questions that warrant further study remain regarding the device, the modality, and the optimal timing of initiation.
Fluid Balance in Critical Care
In ECMO patients, fluid management is critical to prevent complications such as fluid overload, which can lead to oedema, hypoxaemia, and increased mortality. Particularly, positive fluid balance is associated with increased mortality, especially in patients with underlying cardiorespiratory and renal disease (Malbrain et al. 2014; Neyra et al. 2016). Several factors are responsible for fluid overload and the consequences are multisystemic. Therefore, the restoration of euvolaemia is an essential goal in intensive care settings (Kim et al. 2022). FO is also proven to increase the length of mechanical ventilation, the incidence of AKI, the need of CRRT, and the risk of infection and of intra-abdominal hypertension (Salahuddin et al. 2017). AKI, oligo-anuria, positive pressure mechanical ventilation, stress response retention of sodium and water, abdominal compartment syndrome, and iatrogenic simultaneous fluid loading are potential risk factors of FO (Wang et al. 2015). Concurrently, an inadequate fluid balance evaluation and an insufficient fluid unloading strategy may even worse fluid retention.
The consequence of excessive fluid administration and retention leads to an expansion of interstitial space and increased venous congestion. The resulting elevated mean circulatory filling pressure, and the altered transmural pressure of the circulatory system leads to tissue oedema, severe hypoalbuminaemia, inflammatory capillary leakage and impaired lymphatic drainage that in turn may impair intra-abdominal pressure, cardiac function and pulmonary gas exchange, and also reduce lung compliance, increases the work of breathing, potentially leading to a multiorgan failure (Monnet and Teboul 2018). Additionally, venous congestion and increased intraabdominal pressure are well recognised risk factors of AKI development, with a huge impact of fluid removal. Increased venous pressure alters renal blood flow with consequent inadequate glomerular filtration rate (Doty et al. 1999).
In order to tailor fluid balance to the needs of patients, it is also crucial to set specific treatment strategies in different clinical situations (e.g., resuscitative vs. post-resuscitative phase) to prevent or treat FO (Ramesh et al. 2019). In an intensive care setting, specific patient demands can vary quickly, and sometimes conflicting requirements may coexist (Malbrain et al. 2018). Indeed, in the acute “resuscitative” phase, fluid administration is mandatory to reach haemodynamic goals. Even more, large amounts of fluid are often required in order to meet nutrition demand or drug dosage targets. In all these situations, CRRT may represent an important aid, allowing the continuous manipulation of net fluid removal. Even if there is not a solid conclusion about the optimal timing of initiation and CRRT modality, CRRT may allow clinicians to meet the dynamic changes in a patient’s fluid requirement (Prowle and Mehta 2021). Clinicians have to evaluate at fixed intervals, the total volume of fluid that is essential to be removed, in the light of fluid administration required to meet specific needs, and of haemodynamic and volume status (Neyra et al. 2022). In order to provide precise fluid management therapy, CRRT can regulate, not only the total volume of fluid removal, but also the rate of fluid removal (Murugan et al. 2016). This is particularly important in haemodynamic unstable critical care patients, also to maintain a physiological plasma refilling rate.
Finally, another important aspect to consider is the fact that fluid administration is important for the maintenance of the patency of the CRRT circuit itself. Fluids administered before the filter (pre-dilution) can maintain circuit integrity and prevent clotting formation. Fluid balance can be achieved modifying the ultrafiltration rate and the replacement fluid, keeping in mind that the variation of the effluent volume will affect solute clearance (dose) (Claure-Del Granado and Clark 2021). Continuous monitoring of circuit integrity is essential for optimal delivery of CRRT and patient safety.
Fluid Status Assessment in ECMO Patients
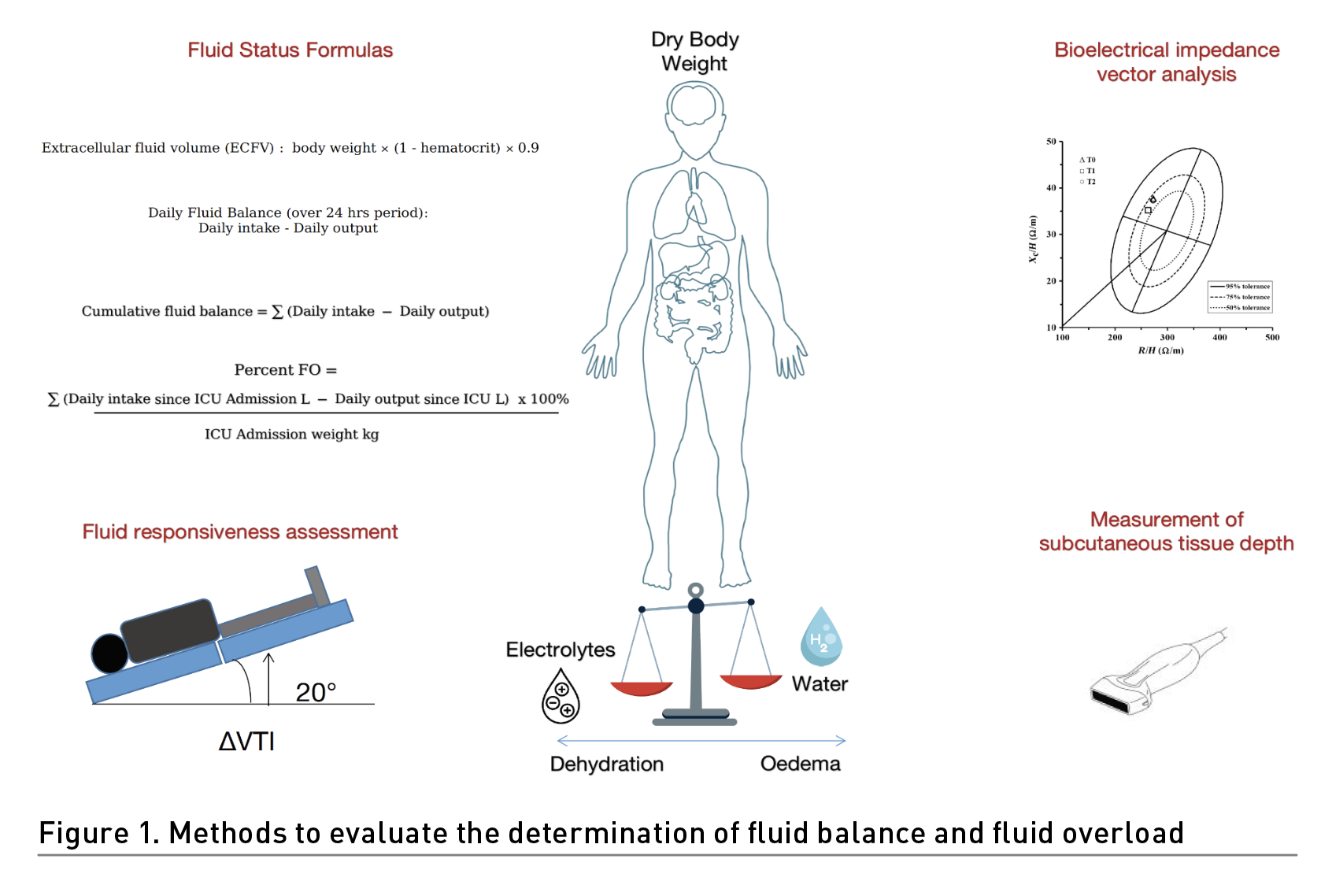
Fluid status assessment is an essential part of the management of patients undergoing ECMO therapy (Freitag et al. 2010). Although the clinical examination includes a physical examination of the patient for signs of fluid overload, precise fluid assessment and recording is extremely challenging in a critically ill setting. Daily weights could be an essential tool for fluid status assessment in patients with extracorporeal multiorgan support, but it is necessary to record the “dry body weight” (i.e., weight before fluid resuscitation) with a goal to return from the extracellular volume to normal (dry weight).
There are several formulas that can be used to assess fluid status in ECMO patients. Fluid balance can be calculated by subtracting total fluid output (urine output, insensible losses, drainage) from total fluid input (oral, intravenous, enteral). Net fluid balance could be calculated by subtracting the amount of fluid removed by ultrafiltration or haemofiltration from total fluid input. This formula can help assess the effectiveness of fluid removal therapy. In addition, cumulative fluid balance could be assessed as the sum of daily fluid balance over a defined period, such as 24 hours or 48 hours. This formula can help assess trends in fluid status over time. Extracellular fluid volume (ECFV) could be also determined by body weight×(1-haematocrit)×0.9. This formula assumes that the haematocrit is a good estimate of the intravascular volume. It is important to note that these formulas should be used in conjunction with clinical examination and other monitoring methods to assess fluid status accurately in ECMO patients. However, haemodynamic monitoring can help determine fluid status and guide fluid management.
Indeed, predicting fluid responsiveness is important in avoiding unnecessary fluid administration, reducing the risk of renal failure, and improving outcomes for critically ill patients. Although pulse pressure variations and stroke volume variations accurately predict fluid responsiveness during mechanical ventilation, unfortunately there is scarce evidence on fluid responsiveness assessments in patients with ECMO (Yang and Du 2014; Jozwiak et al. 2018). Luo et al. (2021) found that changes in left ventricular outflow tract velocity-time integral (ΔVTI) induced by the Trendelenburg manoeuvres could effectively predict fluid responsiveness in VA-ECMO patients.
Bedside ultrasound can be used to evaluate fluid status in ECMO patients. Non-invasive ultrasonographic assessment of skin tissue thickness seems to give further information to identify fluid shifts to the extravascular space and to guide fluid management (Sarvazyan et al. 2005; Wagner and Cotter 2021). The evaluation of the interstitial thickness measuring the distance between superficial dermis surface and the bone tissue interface in the calcaneus area with a linear array transducer is one of the proposed methods to determine hydration status. The measurement of subcutaneous tissue depth between the skin surface to the adipose-muscle boundary in four different body areas (i.e., upper anterior chest, lateral chest, lateral abdomen and anterior tight) could be another tool to estimate FB. These methods, even if particularly interesting, still require further evaluation.
Obviously, point of care ultrasound (POCUS) to assess volume status have gained a huge role in fluid management for intensive care specialists in the last years (Argaiz et al. 2021). However, despite the assessment of inferior vena cava collapsibility and the presence of pleural effusions that can help identify fluid overload, the use of VV-ECMO and VA-ECMO always entails presence of semi-rigid central venous cannula(s) occupying the inferior vena cava (IVC) to a variable extent, thereby limiting its collapsibility (Via et al. 2016).
In addition, the negative venous pressure that can interfere with the IVC size and respiratory dynamics, the masses compressing/occupying the vessel, vena cava filters or IVC thrombosis can equally affect physiological IVC patency and size. However, a deep description of the role of LUS and ultrasonographic haemodynamic evaluation (i.e., inferior vena cava collapsibility index – IVC CI) for fluid management is beyond the scope of this paper.
Bioelectrical impedance vector analysis (BIVA) may represent a viable promising tool that involves the measurement of electrical resistance to assess body composition, including extracellular fluid volume (Samoni et al. 2016; Basso et al. 2013). Although BIVA can reflect the fluid overload state earlier and bypass errors due to fluid balance accounting, it is not well investigated in both ECMO and integrated systems settings (Wang et al. 2021).
Fluid Overload in ECMO Patients
Due to the severity of the underlying disease and to the intrinsic characteristic of the circuit, patients on ECMO may receive a large volume of crystalloids and blood products (Chiu et al. 2021). In order to maintain a sufficient rate of vascular blood drainage for ECMO flow, clinicians often administered large volumes of fluid especially during the initial phases of ECMO. Even more, due to the several complications that may arise during the treatment (e.g., bleeding, anaemia, coagulopathies), patients also receive an important amount of blood products. Fluid administration is important for the maintenance of the patency of the ECMO circuit and to prevent premature circuit changes. This liberal approach of fluid infusion during ECMO exacerbated the underlying disease, often characterised by systemic hypovolaemic status. Even more, blood exposure to the artificial surfaces of ECMO circuit, can worsen systemic capillary leakage, with consequent increase in interstitial and tissue oedema. To make things worse, the high concomitant prevalence of AKI in ECMO patients, reducing fluid output, aggravates fluid overload (Cheng et al. 2014).
Fluid overload can exacerbate the underlying cardiopulmonary disease and prolong cardiorespiratory recovery and time to ECMO weaning. FO during ECMO has been associated with prolonged ECMO duration and mortality (Selewski et al. 2017). Another important aspect that emerges from the existing literature is that a percentage of ECMO patients (up to 50%) present FO before ECMO cannulation and that higher level of FO at CRRT beginning is associated with increased mortality and ECMO duration. Consequently, pre-ECMO fluid balance represents an important target intervention. Even more, He et al. (2018) found that fluid balance on the third day of ECMO initiation and lactate level at CRRT beginning both represent prognosis independent risk factors for patients undergoing CRRT while on ECMO. Therefore, even if volume resuscitating strategy is fundamental especially during initiation of ECMO treatment, excessive volume overload impacts survival and outcome (Schmidt et al. 2014). However, to find a specific threshold is still challenging. From this perspective, the possible correlation between specific threshold of fluid balance (mL/kg) and mortality warrants further studies (Kim et al. 2018). Even more, it will be interesting to evaluate if the threshold diverges according to the indication for ECMO treatment (i.e., cardiovascular versus respiratory disease).
The prevention or the treatment of FO in these kinds of patients can require aggressive use of diuretics with potential collateral effects or fluid restriction with consequent reducing ideal caloric intake. Consequently, the addition of CRRT during ECMO can diminish the administration of diuretics and allow a precise nutritional target. However, also in this group of patients, open questions still exist on the optimal timing, dose prescription target, and ideal modality technique (Paek et al. 2018).
However, FO is a common complication in paediatric patients receiving ECMO therapy, and it can have serious consequences such as pulmonary oedema, decreased oxygen delivery, and increased mortality. Over 75% of patients had a positive fluid balance while on ECMO, suggesting that FO and the ability to achieve a negative fluid balance are potentially important therapeutic targets (Selewski et al. 2017; Sakurai and Singhal 2022). The ability to achieve a negative fluid balance on ECMO is associated with improved survival. CRRT provides flexibility and control in fluid management and has been shown to enhance the ability to achieve dry weight and negative fluid balance during ECMO (Rajapreyar et al. 2021).
Taken together with the epidemiology of FO and its independent association with adverse outcomes in this study, these results suggest that a trial utilising CRRT to manage fluids in children on ECMO may be warranted and probably as a need for earlier intervention. While these studies provide some evidence for the use of ECMO-CRRT integrated systems in managing fluid overload in the paediatric population, larger, randomised controlled trials are needed to establish the safety and efficacy of this approach. In the meantime, fluid management should be closely monitored in paediatric patients on ECMO, and a multidisciplinary team approach should be taken to optimise patient outcomes. Table 1 shows studies evaluating fluid overload and integrated systems in critically ill patients.
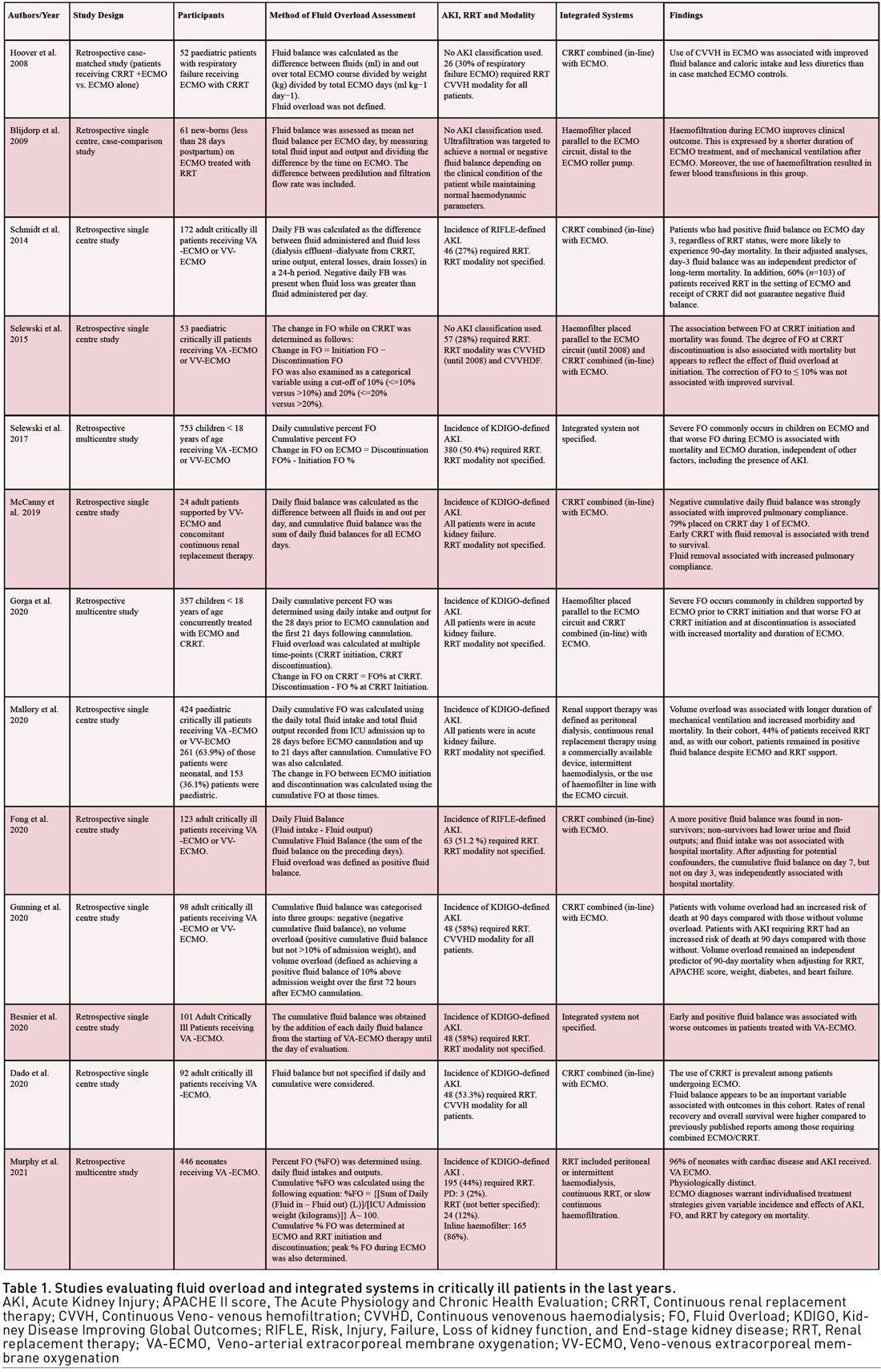
Indications and Modality for Integrated Systems
Indications for ECMO-CRRT integrated systems are represented by severe acute respiratory distress syndrome (ARDS) and AKI who require both respiratory and renal support, sepsis or multi-organ failure who require simultaneous ECMO and CRRT therapy, cardiac arrest who require ECMO and CRRT for haemodynamic and metabolic stabilisation.
During ECMO, RRT can be provided by introducing a haemofilter or RRT circuit into the ECMO circuit (integrated system) or independently via a separate catheter and circuit (parallel system) (Chen et al. 2014; Kielstein et al. 2013). Parallel systems are not the objective of the present paper.
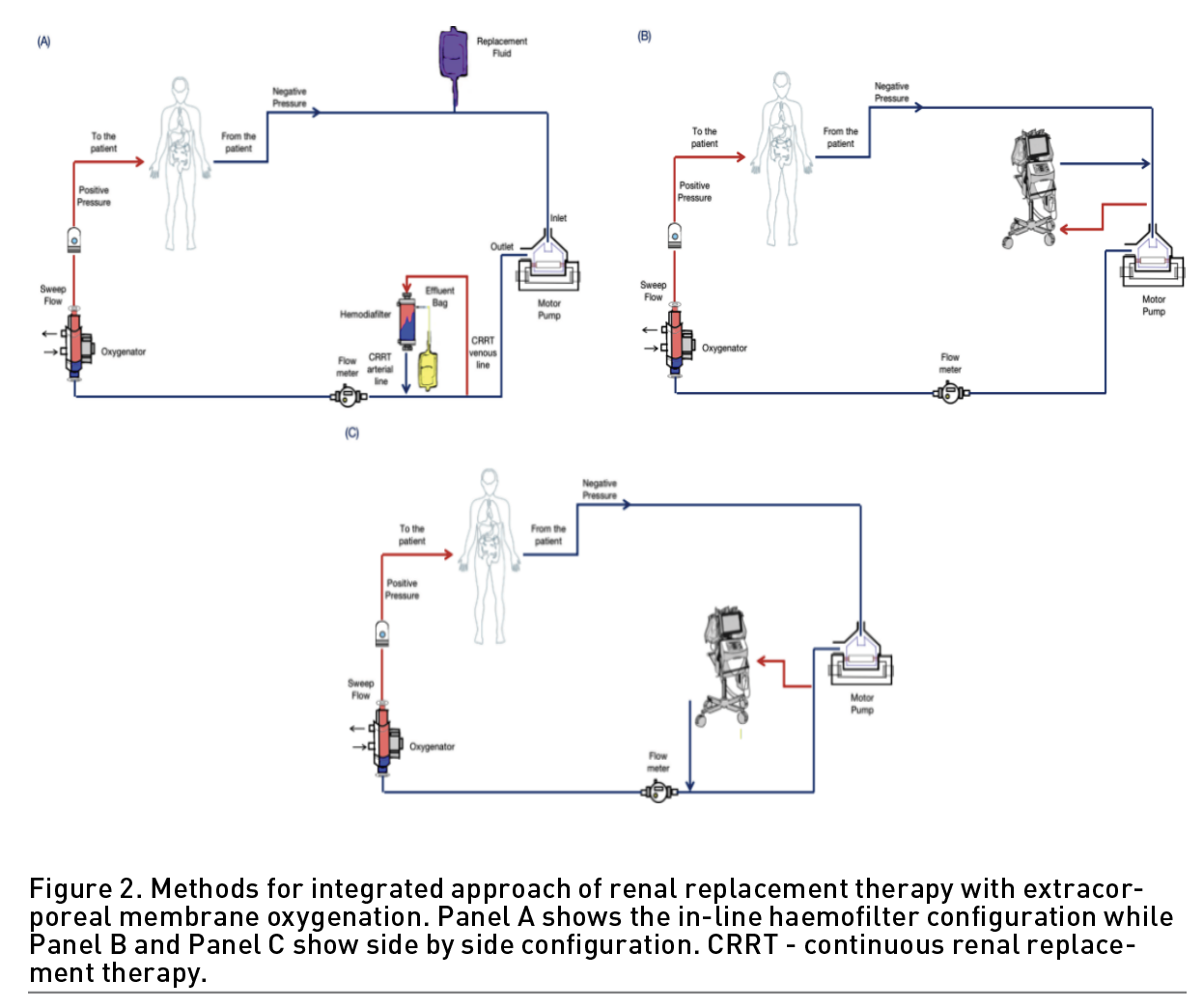
The ECMO-CRRT integrated systems are typically configured in three different ways as following:
- In-line haemofilter (Figure 2, Panel A): In this modality, the CRRT circuit is connected in-line with the ECMO circuit, allowing for continuous ultrafiltration and dialysis. The filter inlet is typically connected after the oxygenator in the ECMO circuit to avoid interference with gas exchange and the outlet is reconnected to the ECMO circuit to allow the return of the blood before it enters the oxygenator. The blood from ECMO circuit is shunted through in-line haemofilter. The intravenous pump allows the control of fluid removal. The fluid replacement or dialysis fluid can allow an additional solute clearance.
- Side-by-side (Figure 2, Panel B and C): In this modality, the ECMO and CRRT circuits are arranged side-by-side, with a common venous access and a common arterial return, also possible via existing Luer locks on the inlet and outlet ports of the oxygenator. It requires a larger footprint and more complex tubing arrangement. There is no need for separate vascular access and anticoagulation. This modality allows the control of pressures inside the circuit but also for higher ultrafiltration rates without the need of an external pump with better clearance of solutes (Chen et al. 2014). However, not all devices are able to recognise pressure changes leading to iterative stops and interruptions in treatment. In addition, the integration after ECMO motor pump could evoke high pressure alarms (de Tymowski et al. 2017). Therefore, since the two integrated circuits have two different pressure levels, the pressure differences can cause extremely risky situations with a shunt effect inside the ECMO circuit. The integration could be possible, and it is strongly suggested that the blood return to the ECMO circuit before the oxygenator.
The modality chosen should depend on the patient's specific needs and the available resources. The use of ECMO-CRRT integrated systems requires expertise in both ECMO and CRRT therapies and should only be performed by trained healthcare professionals.
Effects of Integrated Systems on Outcome
There is no strong recommendation on the optimal RRT modality during ECMO and the decision depends on local expertise and availability. The role of CRRT in modifying the outcome in ECMO patients is still uncertain.
Several published articles found that the mortality was high in patients receiving ECMO and CRRT in comparison with ECMO alone (Chen et al. 2014; Mitra et al. 2021; Han et al. 2015). However, it is important to stress that this data has to be interpreted with caution. First, the impact of the severity of AKI itself may contribute to the increased mortality in such fragile patients. Even more, AKI requiring RRT is associated with other life-treating complications (e.g., sepsis, immunodeficiency, hepatic failure, bleeding, neurological complications), increasing the severity of the underlying disease of ECMO patients. Nevertheless, the high heterogeneity of initiation, FO evaluation modalities and the differences in AKI definitions may have to be considered. Furthermore, there is no clear evidence that the different strategies (i.e., parallel, integrated) may impact mortality or ECMO duration. Noteworthy, the aetiology of AKI in patients on ECMO is multifactorial, consequently, even if the studies are matched for severity of illness, it is difficult to come to any solid conclusions (Martins Costa et al. 2022). Central risk factors for mortality in ECMO patients with AKI are represented and not limited to age, AKI stage, RRT duration, hypercapnia, multiorgan failure syndrome, blood loss, transfusion requirement, haemodynamic instability, liver failure, and fluid overload (Ostermann and Lumlertgul 2021).
Not only the short-term outcome but also long-term outcomes and renal recovery are other important aspects that warrant major attention. Up to now, the renal recovery, the rate of liberation from dialysis, chronic kidney disease rate and quality of life is still indeterminate and require major evaluation in future trials. What seems to emerge from the existing literature is that lower GFR at baseline, higher AKI stage prior ECMO cannulation, transfusion requirement represents risk factor for 1-year major adverse kidney events. Consequently, the risk of kidney events in ECMO survivors has to be precisely evaluated (Ostermann and Lumlertgul 2021).
Complications related to CRRT during ECMO may arise during vascular placement of the cannula (e.g., bleeding, pneumothorax, haemothorax, retroperitoneal haemorrhage, vascular injury, arterial puncture, fistula formation) and during the treatment itself (e.g., infection, thrombosis, arrhythmias, hypokalaemia, hypophosphataemia, hypothermia, nutrient losses, haemolysis, intracranial haemorrhage and gastrointestinal bleeding). Special attention has to be put on the prevention of air embolism that can arise in several procedures during the treatment (e.g., central line insertion, connecting/disconnecting CRRT or infusions and flow variations due to the interaction of pressure and flow of ECMO and CRRT system). Another vital aspect is represented by a tight control of the anticoagulation regimen (e.g., systemic, regional) in order to prevent thrombosis, bleeding, premature circuit clotting and to increase circuit patency (Selewski and Wille 2021). However, in a 2014 systematic review, the authors found that the integration of ECMO and CRRT systems appear to be safe and effective with improvement in fluid removal and electrolyte disturbances (Chen et al. 2014).
Technical Issues
Each specific modality system to perform CRRT during ECMO presents specific advantages and drawbacks that need to be known before initiation of the treatment. A deep understanding of intra-circuit pressure and flow is essential to guarantee safety during the treatment (Ostermann et al. 2018; Wu et al. 2023; Na et al. 2018).
An integrated system is characterised by the introduction of an in-line haemofilter or a CRRT machine into the ECMO circuit. The introduction of a haemofilter requires a smaller priming volume compared to a parallel approach. However, ECMO circuit is characterised to work with negative pressure in the drainage part of the circuit (e.g., -20 to -100mmHg) and with positive pressure between the pump and oxygenator and between the oxygenator and the patients (Kashani and Ostermann 2019). Conversely, the CRRT machine generally works with venous pressure (from and to the patients) from about o to 30 mmHg. Furthermore, the flow within ECMO circuits is higher (3500-5000ml/min) than those working on a CRRT system (100-200mL/min). Possible complications of pressure and flows differences are represented by air entrapment, turbulences, haemolysis, increasing shear stress and alarms out of range. Another important implication of an in-line system is represented by the difficulty of the net ultrafiltration evaluation due to the fact that part in-line system required an external infusion pump and the presence of tube ramification within the circuits (Askenazi et al. 2012). This led to an important difference between prescribed and actual ultrafiltration rate. Using a CRRT machine within the ECMO circuit can obviate of this issue (de Tymowski et al. 2017). A CRRT circuit allows to control pressure, ultrafiltration without an external pump with a more precise control of effluent volume (Santiago et al. 2009). However, also in this setting, problems with pressure alarms and connection lines may arise with consequent interruption in the treatment or complications such as air embolism and flow turbulence. Not to be underestimated, the introduction of an integrated system within the ECMO system with tube ramification is responsible of blood shunt off ECMO circuit with consequent potential alteration in oxygenation and blood flow.
Of course, such technical aspects and possible complications are not encountered in parallel systems, characterised by an independent circuit to deliver either ECMO or CRRT modalities (Martins Costa et al. 2022). In the parallel approach, no interferences are encountered between ECMO and CRRT techniques (Seczyńska et al. 2014). CRRT can be prescribed and monitored independently from ECMO and not to be underestimated, CRRT changing can be accomplished with less risk and without the contribution of an ECMO expert. However, a separate vascular access is required, with possible consequent bleeding, infection and thrombosis risk (Subbarayan et al. 2021). Even more, the use of a vascular site for CRRT may diminish the choice of access site in case of the necessity of an additional cannula for a higher output of ECMO treatment. Furthermore, the usage of an independent circuit increases the artificial surface with increased risk of activation of coagulation cascade, systemic inflammation, shear stress and haemolysis.
In order to obviate the aforementioned issue, novel extracorporeal devices are currently under development, with particular attention on fibre arrangement, filtration mode, artificial surface characteristics and connections (Tang et al. 2022).
Conclusions
The successful delivery of CRRT during ECMO requires a clear prescription of the target solute clearance and fluid removal rate based on the cumulative fluid balance and physiological variables (haemodynamic, oxygenation). Treatment monitoring and re-adjustment are necessary and are based on patients need. While the deleterious impact of AKI and FO on outcomes for ECMO patients is clear, critical questions warranting further study remain regarding the role of CRRT in patient management, including device, modality, and optimal timing of initiation.
Conflict of interest
None.
References:
Argaiz ER, Koratala A, Reisinger N (2021) Comprehensive Assessment of Fluid Status by Point-of-Care Ultrasonography. Kidney360. 2(8):1326-38.
Askenazi DJ, Selewski DT, Paden ML et al. (2012) Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. 7(8):1328-36.
Basso F, Berdin G, Virzì GM et al. (2013) Fluid management in the intensive care unit: bioelectrical impedance vector analysis as a tool to assess hydration status and optimal fluid balance in critically ill patients. Blood Purif. 36(3-4):192-9.
Besnier E, Boubèche S, Clavier T et al. (2020) Early Positive Fluid Balance is Associated with Mortality in Patients Treated with Veno-Arterial Extra Corporeal Membrane Oxygenation For Cardiogenic Shock: a Retrospective Cohort Study. Shock. 53(4):426-33.
Blijdorp K, Cransberg K, Wildschut ED et al. (2009) Gischler SJ, Jan Houmes R, Wolff ED, et al. Haemofiltration in newborns treated with extracorporeal membrane oxygenation: a case-comparison study. Crit Care. 13(2):R48.
Chen H, Yu RG, Yin NN, Zhou JX (2014) Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit Care. 18(6):675.
Cheng R, Hachamovitch R, Kittleson M et al. (2014) Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. 97(2):610-6.
Chiu LC, Chuang LP, Lin SW et al. (2021) Chiou YC, Li HH, Chen YC, et al. Cumulative Fluid Balance during Extracorporeal Membrane Oxygenation and Mortality in Patients with Acute Respiratory Distress Syndrome. Membranes (Basel). 11(8).
Claure-Del Granado R, Clark WR (2021) Continuous renal replacement therapy principles. Semin Dial. 34(6):398-405.
Dado DN, Ainsworth CR, Thomas SB et al. (2020) Outcomes among Patients Treated with Renal Replacement Therapy during Extracorporeal Membrane Oxygenation: A Single-Center Retrospective Study. Blood Purif. 49(3):341-7.
de Tymowski C, Augustin P, Houissa H et al. (2017) CRRT Connected to ECMO: Managing High Pressures. ASAIO J. 2017;63(1):48-52.
Doty JM, Saggi BH, Sugerman HJ et al. (1999) Effect of increased renal venous pressure on renal function. J Trauma. 47(6):1000-3.
Eckman PM, Katz JN, El Banayosy A et al. (2019) Veno-Arterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock: An Introduction for the Busy Clinician. Circulation. 140(24):2019-37.
Fleming GM, Askenazi DJ, Bridges BC et al. (2012) A Multicenter International Survey of Renal Supportive Therapy During ECMO: The Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) Group. ASAIO J. 58(4):407-14.
Fong KM, Au SY, Ng GWY, Leung AKH (2020) Positive fluid balance and mortality in adult patients treated with extracorporeal membrane oxygenation: A retrospective study. J Intensive Care Soc. 21(3):210-20.
Freitag E, Edgecombe G, Baldwin I (2010) Cottier B, Heland M. Determination of body weight and height measurement for critically ill patients admitted to the intensive care unit: A quality improvement project. Aust Crit Care. 23(4):197-207.
Gorga SM, Sahay RD, Askenazi DJ et al. (2020) Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy: a multicenter retrospective cohort study. Pediatr Nephrol. 35(5):871-82.
Gu M, Mei XL, Zhao YN (2021) A review on extracorporeal membrane oxygenation and kidney injury. J Biochem Mol Toxicol. 35(3):e22679.
Gunning S, Kutuby F, Rose R et al. (2020) Fluid Overload and Mortality in Patients with Severe Acute Kidney Injury and Extracorporeal Membrane Oxygenation. Kidney360. 1(4):232-40.
Han SS, Kim HJ, Lee SJ et al. (2015) Kim WJ, Hong Y, Lee HY, et al. Effects of Renal Replacement Therapy in Patients Receiving Extracorporeal Membrane Oxygenation: A Meta-Analysis. Ann Thorac Surg. 100(4):1485-95.
He P, Zhang S, Hu B, Wu W (2018) Retrospective study on the effects of the prognosis of patients treated with extracorporeal membrane oxygenation combined with continuous renal replacement therapy. Ann Transl Med. 6(23):455.
Hoover NG, Heard M, Reid C et al. (2008) Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med. 34(12):2241-7.
Jozwiak M, Monnet X, Teboul JL (2018) Prediction of fluid responsiveness in ventilated patients. Ann Transl Med. 6(18):352.
Kalantari K, Chang JN, Ronco C, Rosner MH (2013) Assessment of intravascular volume status and volume responsiveness in critically ill patients. Kidney Int. 83(6):1017-28.
Kashani K, Ostermann M (2019) Optimizing renal replacement therapy for patients who need extracorporeal membrane oxygenation: crosstalk between two organ support machines. BMC Nephrol. 20:404.
Kielstein JT, Heiden AM, Beutel G et al. (2013) Gottlieb J, Wiesner O, Hafer C, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant. 28(1):86-90.
Kim KY, Ryu JH, Kang DH et al. (2022) Early fluid management affects short-term mortality in patients with end-stage kidney disease undergoing chronic hemodialysis and requiring continuous renal replacement therapy. BMC Nephrol. 23.
Kim H, Paek JH, Song JH et al. (2018) Lee H, Jhee JH, Park S, et al. Permissive fluid volume in adult patients undergoing extracorporeal membrane oxygenation treatment. Crit Care. 22(1):270.
Luo JC, Su Y, Dong LL et al. (2021) Trendelenburg maneuver predicts fluid responsiveness in patients on veno-arterial extracorporeal membrane oxygenation. Ann Intensive Care. 11(1):16.
Malbrain ML, Marik PE, Witters I et al. (2014) Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 46(5):361-80.
Malbrain M, Van Regenmortel N, Saugel B et al. (2018) Principles of fluid management and stewardship in septic shock: it is time to consider the four D's and the four phases of fluid therapy. Ann Intensive Care. 8(1):66.
Mallory PP, Selewski DT, Askenazi DJ et al. (2020) Acute Kidney Injury, Fluid Overload, and Outcomes in Children Supported With Extracorporeal Membrane Oxygenation for a Respiratory Indication. ASAIO J. 66(3):319-26.
Martins Costa A, Halfwerk F, Wiegmann B et al. (2022) Trends, Advantages and Disadvantages in Combined Extracorporeal Lung and Kidney Support From a Technical Point of View. Front Med Technol. 4:909990.
McCanny P, Smith MW, O'Brien SG et al. (2019) Fluid Balance and Recovery of Native Lung Function in Adult Patients Supported by Venovenous Extracorporeal Membrane Oxygenation and Continuous Renal Replacement Therapy. ASAIO J. 65(6):614-9.
Mitra S, Ling RR, Tan CS et al. (2021) Concurrent Use of Renal Replacement Therapy during Extracorporeal Membrane Oxygenation Support: A Systematic Review and Meta-Analysis. J Clin Med. 10(2).
Monnet X, Teboul JL (2018) My patient has received fluid. How to assess its efficacy and side effects? Ann Intensive Care. 8(1):54.
Murphy HJ, Gien J, Sahay R et al. (2021) Acute Kidney Injury, Fluid Overload, and Renal Replacement Therapy Differ by Underlying Diagnosis in Neonatal Extracorporeal Support and Impact Mortality Disparately. Blood Purif. 50(6):808-17.
Murugan R, Hoste E, Mehta RL et al. (2016) Precision Fluid Management in Continuous Renal Replacement Therapy. Blood Purif. 42(3):266-78.
Na SJ, Choi HJ, Chung CR et al. (2018) Using additional pressure control lines when connecting a continuous renal replacement therapy device to an extracorporeal membrane oxygenation circuit. BMC Nephrol. 19(1):369.
Neyra JA, Li X, Canepa-Escaro F et al. (2016) Cumulative Fluid Balance and Mortality in Septic Patients with or without Acute Kidney Injury and Chronic Kidney Disease. Crit Care Med. 44(10):1891-900.
Neyra JA, Lambert J, Ortiz-Soriano V et al. (2022) Assessment of prescribed vs. achieved fluid balance during continuous renal replacement therapy and mortality outcome. PLoS One. 17(8):e0272913.
Ostermann M, Lumlertgul N (2021) Acute kidney injury in ECMO patients. Crit Care. 25(1):313.
Ostermann M, Straaten HM, Forni LG (2015) Fluid overload and acute kidney injury: cause or consequence? Crit Care. 19:443.
Ostermann M, Connor M Jr., Kashani K (2018) Continuous renal replacement therapy during extracorporeal membrane oxygenation: why, when and how? Curr Opin Crit Care. 24(6):493-503.
Paek JH, Park S, Lee A et al. (2018) Chin HJ, Na KY, Lee H, et al. Timing for initiation of sequential continuous renal replacement therapy in patients on extracorporeal membrane oxygenation. Kidney Res Clin Pract. 37(3):239-47.
Patil VP, Salunke BG (2020) Fluid Overload and Acute Kidney Injury. Indian J Crit Care Med. 24(Suppl 3):S94-7.
Prowle J, Mehta R (2021) Fluid balance management during continuous renal replacement therapy. Semin Dial. 34(6):440-8.
Rajapreyar P, Castaneda L, Thompson NE et al. (2021) Association of Fluid Balance and Survival of Pediatric Patients Treated With Extracorporeal Membrane Oxygenation. Front Pediatr. 9:722477.
Ramesh GH, Uma JC, Farhath S (2019) Fluid resuscitation in trauma: what are the best strategies and fluids? Int J Emerg Med. 12(1):38.
Sakurai K, Singhal N (2022) Extracorporeal membrane oxygenation in children: A brief review. J Paediatr Child Health. 58(9):1525-31.
Salahuddin N, Sammani M, Hamdan A et al. (2017) Fluid overload is an independent risk factor for acute kidney injury in critically Ill patients: results of a cohort study. BMC Nephrol. 18.
Samoni S, Vigo V, Reséndiz Li et al. (2016) Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit Care. 20:95.
Santiago MJ, Sánchez A, López-Herce J et al. (2009) Pérez R, del Castillo J, Urbano J, et al. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int. 76(12):1289-92.
Sarvazyan A, Tatarinov A, Sarvazyan N (2005) Ultrasonic assessment of tissue hydration status. Ultrasonics. 43(8):661-71.
Schmidt M, Bailey M, Kelly J et al. (2014) Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 40(9):1256-66.
Seczyńska B, Królikowski W, Nowak I et al. (2014) Continuous renal replacement therapy during extracorporeal membrane oxygenation in patients treated in medical intensive care unit: technical considerations. Ther Apher Dial. 18(6):523-34.
Selewski DT, Askenazi DJ, Bridges BC et al. (2017) The Impact of Fluid Overload on Outcomes in Children Treated With Extracorporeal Membrane Oxygenation: A Multicenter Retrospective Cohort Study. Pediatr Crit Care Med. 18(12):1126-35.
Selewski DT, Wille KM (2021) Continuous renal replacement therapy in patients treated with extracorporeal membrane oxygenation. Semin Dial. 34(6):537-49.
Selewski DT, Cornell TT, Blatt NB et al. (2012) Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 40(9):2694-9.
Silversides JA, Major E, Ferguson AJ et al. (2017) Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 43(2):155-70.
Subbarayan B, Vivek V, Kuppuswamy MK (2021) Renal replacement therapy during extracorporeal membrane oxygenation. Indian J Thorac Cardiovasc Surg. 37(Suppl 2):261-6.
Tandukar S, Palevsky PM (2019) Continuous Renal Replacement Therapy: Who, When, Why, and How. Chest. 155(3):626-38.
Tang YS, Tsai YC, Chen TW, Li SY (2022) Artificial Kidney Engineering: The Development of Dialysis Membranes for Blood Purification. Membranes (Basel). 12(2).
Via G, Tavazzi G, Price S (2016) Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: a physiologically based point of view. Intensive Care Med. 42(7):1164-7.
Wagner DR, Cotter JD (2021) Ultrasound Measurements of Subcutaneous Fat Thickness Are Robust Against Hydration Changes. Int J Sport Nutr Exerc Metab. 31(3):244-9.
Wang N, Jiang L, Zhu B et al. (2015) Wen Y, Xi XM. Fluid balance and mortality in critically ill patients with acute kidney injury: a multicenter prospective epidemiological study. Crit Care. 19.
Wang K, Sun SL, Wang XY et al. (2021) Chu CN, Duan ZH, Yang C, et al. Bioelectrical impedance analysis-guided fluid management promotes primary fascial closure after open abdomen: a randomized controlled trial. Mil Med Res. 8(1):36.
Wu J, Huang X, Mei Y et al. (2023) Impact of connecting methods of continuous renal replacement therapy device on patients underwent extracorporeal membrane oxygenation: A retrospectively observational study. Aust Crit Care.
Yang X, Du B (2014) Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit Care. 18(6):650.









