ICU Management & Practice, Volume 16 - Issue 4, 2016
Cardiac biomarkers, including natriuretic peptides and troponins, have become widely used in the treatment of heart failure and acute coronary syndrome. As we learn more about the function of these markers, their use has begun to expand. We can now track and utilise natriuretic peptides throughout hospital admission to monitor progress of heart failure therapy. Troponins and natriuretic peptides can provide useful prognostic data and help stratify more high-risk patients. Novel biomarkers, such as ST2, can also aid in prognostication, and may be beneficial in guiding initiation of therapies that reduce cardiac remodelling, including beta-adrenergic receptor and mineralocorticoid receptor antagonists. Finally, procalcitonin can help distinguish dyspnoea secondary to heart failure from pulmonary infection and can help guide use of antibiotics in patients with heart failure who present with shortness of breath.
Heart failure (HF) is the leading cause of mortality in the United States (Lloyd-Jones et al. 2009). Advances in medical therapies have improved outcomes for patients with heart failure with reduced ejection fraction (HFrEF), but these patients still account for over 1 million hospitalizations annually and generate billions of dollars in healthcare costs (Mozaffarian et al. 2015; Go et al. 2014; Ambrosy et al. 2014). Cardiac biomarkers are noninvasive and inexpensive to measure, and they allow for more accurate and rapid diagnosis of acute heart failure exacerbation in the emergency department (ED), which can reduce rates of hospitalisation and health care costs. Cardiac biomarkers can also improve prognostication and help guide medical therapy for heart failure and this therapeutic guidance may improve patient morbidity and mortality. In this review, we will examine two of the most commonly used cardiac biomarkers in the treatment of heart failure: the natriuretic peptides (NPs) and troponins. We will also discuss two novel cardiac biomarkers: ST2, a marker of cardiac remodelling and fibrosis with prognostic value, and procalcitonin, a marker of inflammation that can help guide treatment of bacterial infections.
Natriuretic Peptides
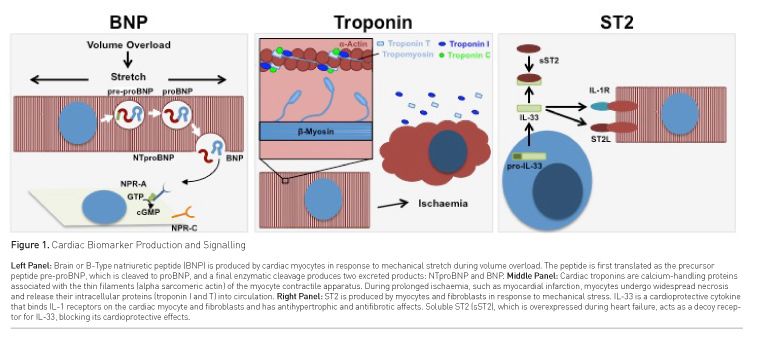
Brain or B-type natriuretic peptides (BNPs) are proteins synthesised by cardiac ventricular myocytes in response to mechanical stretch (Yasue et al. 1994; Yoshimura et al. 1993). At the cellular level in the setting of volume overload, mechanical stretch on cardiomyocyte membranes activates downstream transcription and translation of a 134 amino acid precursor peptide pre-proBNP (Sudoh et al. 1989). This biologically inactive protein undergoes enzymatic cleavage twice: first producing proBNP1-108, and with a second cleavage producing BNP1-32 (the biologically active carboxy-terminal peptide) and the inactive amino terminal fragment NTproBNP (Figure 1, Left Panel). Both peptides are secreted in equimolar amounts into circulation (Daniels and Maisel 2007; Nakagawa et al. 2009; Kojima et al. 1989). Unlike the other natriuretic peptides (Atrial and C-type natriuretic peptides), BNP is minimally stored. It is synthesised and directly secreted in large bursts from the ventricular myocardium (Maisel et al. 2002). NPs act on membrane-bound natriuretic peptide receptors (NPRs) in target tissues to induce vasodilation, diuresis, natriuresis and inhibition of the renin-angiotensin-aldosterone system (RAAS) system. These actions act to reduce cardiac preload and afterload (Daniels and Maisel 2007). BNP is cleared from circulation by binding to NPRs, by degradation by circulating neutral endopeptidases, and to a lesser degree through renal excretion (Daniels and Maisel 2007).
Clinical Use of Natriuretic Peptides
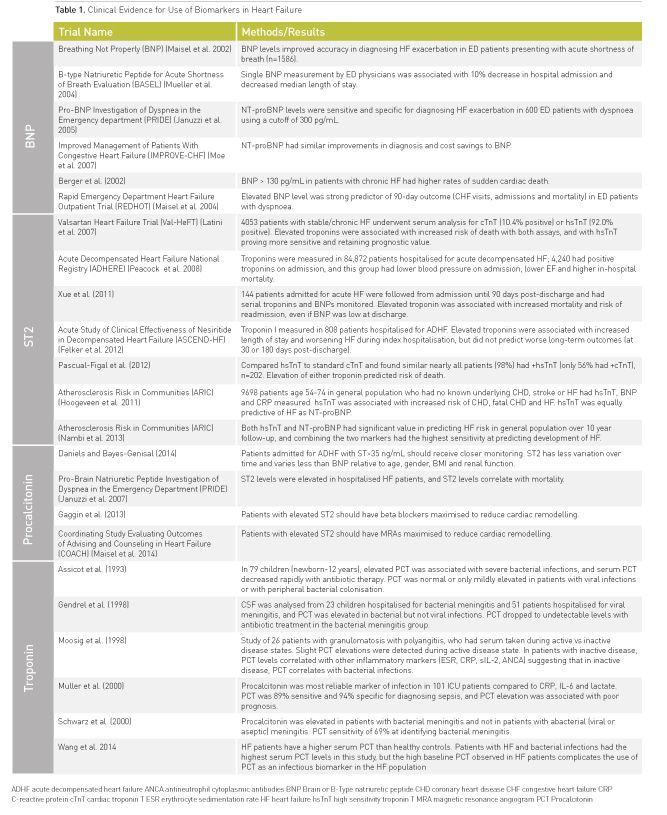
It is now a Class I indication in the American Heart Association / American College of Cardiology (AHA/ACC) guidelines for management of HF that BNP should be measured on hospital admission for all suspected cases of acute HF exacerbation (Yancy et al. 2013). ED providers should utilise BNP levels for risk stratification, with BNP < 400 pg/mL indicating a lower-risk patient that could be safely discharged from the ED with close outpatient follow-up (Maisel et al. 2015; Maisel et al. 2008). Several clinical trials have demonstrated the utility of BNP measurement in the ED (Table 1). In the 2002 Breathing Not Properly multinational study of 1586 patients presenting to the ED with dyspnoea, measurement of serum BNP had higher accuracy in diagnosing heart failure than the ED physicians (Maisel et al. 2002). Using a cutoff of 100 pg/mL, serum BNP was 90% sensitive and 76% specific for heart failure in this trial. The 2005 PRIDE study similarly demonstrated that NT-proBNP was highly sensitive and specific at diagnosing heart failure among 600 ED patients presenting with dyspnoea (Januzzi et al. 2005). This study suggested a cutoff NT-proBNP level of 300 pg/mL to rule out heart failure in these patients. Other studies have demonstrated how measurement of BNP (Mueller et al. 2004) and NT-proBNP (Moe et al. 2007)) in the ED can reduce hospitalisation rates, median length of stay, and thus reduce overall healthcare costs.
BNP has demonstrated prognostic value in both acute (Doust et al. 2005; Maisel et al. 2004) and chronic HF (Berger et al. 2002; Anand et al. 2003), with elevated BNP levels associated with worse outcomes, greater morbidity and higher mortality (Table 1). In both the acute and chronic setting, for every 100 pg/mL increase in BNP, there is a 35% increase in risk of death (Doust et al. 2005). Elevated BNP levels in the ED patient should be considered relative to their last baseline outpatient BNP or from prior to discharge from a previous hospitalisation (Maisel et al. 2015). It may not be necessary to trend NPs daily during the hospitalisation, but serial measurements should be considered in patients who are not clinically improving. BNP values should decrease with diuresis, as studies have demonstrated that in acute decompensated heart failure (ADHF), treatment-related decreases in pulmonary capillary wedge pressure (PCWP) are correlated with a drop in NP levels (Kazanegra et al. 2001). Additionally, failure of NP levels to decrease during a hospitalisation is associated with worse prognosis (Bettencourt et al. 2002; Cheng et al. 2016).
In severely volume-overloaded patients, BNP may not immediately decrease, because fluid volume is initially diuresed primarily from interstitial tissues. So in a severely volume overloaded person, they may diurese several litres initially from their lower extremity or pulmonary interstitium without producing any change in intravascular volume or preload (Wettersten and Maisel 2016). As such, their BNP may not begin to decrease until several days into the hospitalisation when diuresis has begun to affect intravascular volume and cardiac preload. Once intravascular volume and preload begin to decrease, ventricular stretch lessens and BNP production by strained cardiac myocytes begins to decline. With continued diuresis, serum BNP levels will decline towards their baseline/outpatient values. BNP levels should be measured in all patients prior to discharge, as elevated serum BNP at discharge is associated with worse outcomes, including increased readmission rates and mortality, regardless of presenting BNP levels (Dokainish et al. 2005; Logeart et al. 2004). This pre-discharge BNP level may also be used to monitor patients at subsequent outpatient follow up visits (Maisel et al. 2015; Wettersten and Maisel 2016; Maisel 2006).
Caveats of BNP Interpretation
Several factors can cause elevated baseline BNP and NT-proBNP levels, including age (Redfield et al. 2002; Wang et al. 2002; Costello-Boerrigter et al. 2006), female gender (Redfield et al. 2002; Wang et al. 2002; Costello-Boerrigter et al. 2006), and renal dysfunction (Tsutamoto et al. 2006). The higher levels of circulating NPs at baseline with advanced age are independent of age-related diastolic dysfunction (Redfield et al. 2002), and may be due to age-related reduction of NPRs, which results in decreased clearance of circulating NPs (Daniels and Maisel 2007). In these studies, age-matched cohorts demonstrated that BNP and NT-proBNP levels are higher in women than men at any age (Redfield et al. 2002; Wang et al. 2002). Several researchers have proposed that oestrogen levels may be involved, as women on hormone replacement therapy (HRT) had higher BNP levels than women not on therapy (Redfield et al. 2002), although oestrogen replacement had only minimal effects on NT-proBNP levels in this same study (Costello-Boerrigter et al. 2006).
The relationship of BNP to renal function is more complex and likely multifactorial, as BNP is mostly taken up by NPRs or enzymatically degraded in serum rather than cleared renally (McCullough et al. 2003). Older patients with renal dysfunction may have chronically higher intravascular volume, increased ventricular strain and reduced glomerular filtration, which could all contribute to the elevated BNP levels observed in patients with chronic kidney disease.
Low BNP levels may result in obese patients and during early flash pulmonary oedema. The negative correlation between obesity and baseline serum BNP levels is well documented (Wang et al. 2002; Wang et al. 2004; Mehra e al. 2004; Daniels et al. 2006), but the exact mechanism remains unclear. Some have hypothesised that adipocytes have increased concentration of NPRs, which could increase BNP clearance (Sarzani et al. 1996), but others have demonstrated a positive correlation between BNP levels and lean mass rather than fat mass (Das et al. 2005). There is conflicting evidence as to whether NT-proBNP levels are similarly low in obese patients, which could be explained by the fact that NT-proBNP is not cleared through NPRs. Although BNPs are low in obese patients, they still retain their diagnostic and prognostic values if measured relative to a known baseline (Daniels and Maisel 2004). BNP levels may similarly be low early in flash pulmonary oedema. This is due to the insufficient time for BNP gene expression and translation in the setting of rapid interstitial fluid accumulation (Yoshimura et al. 1993).
Troponins
Troponins are calcium-handling proteins integral to excitation-contraction coupling in the cardiac myocyte (Figure 1, Middle Panel) (Sharma et al. 2004; Parmacek and Solaro 2004). Cardiac troponins and other intracellular myocyte proteins are released into circulation after myocardial infarction, in which prolonged ischaemia resulting from coronary artery occlusion causes myocyte necrosis. Detection of cardiac troponins in the blood (Troponin T or I) is useful for diagnosis of acute coronary syndrome (ACS), as troponin I has not been identified in tissues outside the myocardium (Bodor et al. 1995), and troponin T is only minimally expressed in skeletal muscle tissues (Ricchiuti et al. 1998). As these serum markers are specific for myocardial damage (Collinson et al. 2001), they are now considered the gold standard for diagnosis of ACS (Braunwald et al. 2000; Bertrand et al. 2000).
Clinical Utility of Troponins in Heart Failure
The AHA/ACC guidelines recommend checking cardiac troponins in patients presenting with acute heart failure, both for ACS rule out and risk stratification (Yancy et al. 2013). In patients presenting with exertional chest pain and dyspnoea, cardiac troponins must be trended over the first 24 hours of hospitalisation to rule out ACS. In patients with acute decompensated HF without ACS (diagnosed by symptoms, ECG changes, and trending cardiac markers), elevated troponins were highly prognostic in several studies (Table 1). Patients admitted for ADHF who had troponin elevation on admission had higher in-hospital mortality in the Acute Decompensated Heart Failure National Registry (ADHERE) trial (Peacock et al. 2008), with increased length of stay and worsening HF during admission in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial (Felker et al. 2012). Both the older cardiac troponin T detection assay (cTnT) and the newer high-sensitivity troponin T assay (hsTnT) have been shown to be useful for prognostication in patients admitted for ADHF. Troponin elevation is detected only in a minority of heart failure patients using the cTnT assay, but hsTnT can detect low concentrations of troponins in the majority of HF patients, and HF patients with elevations of either cTnT or hsTnT demonstrated increased risk of death (Pascual-Figal et al. 2012; Latini et al. 2007). Another study demonstrated that serial troponin measurement, in addition to serial BNP, can add prognostic value. Patients in this study with the highest serum troponins at discharge had increased mortality and higher risk of readmission (Xue et al. 2011). The Atherosclerosis Risk in Communities (ARIC) study evaluated the use of cardiac biomarkers at predicting risk of developing coronary heart disease and heart failure in a pool of 9698 patients without known CHD, stroke or HF. Patients in the general population with elevated hsTnT had significantly increased risk of developing CHD, fatal CHD and HF over 10 year follow-up, and hsTnT had equivalent predictive value as NT-proBNP for detecting development of HF (Saunders et al. 2011). In a follow-up study, the ARIC study authors demonstrated that in this same general population elevation of both hsTnT and NT-proBNP was even more prognostic for prediction of developing HF at 10 year follow-up than either marker individually (Nambi et al. 2013).
ST2
Growth STimulated expressed gene 2 (ST2) is a receptor for interleukin-33 (IL-33) that is expressed by myocytes and fibroblasts in response to mechanical stress. IL-33 is protective against myocardial hypertrophy and fibrosis in animal models of pressure overload (Schmitz et al. 2005; Kuball et al. 2005). ST2, expressed as membrane-bound (ST2L) or soluble (sST2) isoforms through alternative splicing (Kieser et al. 1995; Yanagisawa et al. 1992; Tominaga 1989), is overexpressed in HF, and sST2 acts as a decoy receptor that binds IL-33 and prevents its cardioprotective actions (Sanada et al. 2007; Weir et al. 2010). There are no prospective clinical trials that have examined the utility of ST2 as a clinical biomarker, but many retrospective and observational studies suggest its function as a prognostic indicator and for driving medical therapy.
Clinical Utility of ST2 in Heart Failure
Patients admitted to the hospital with ADHF should have an sST2 level drawn on admission, and patients with serum levels >35 ng/mL should receive closer monitoring, especially if other clinical biomarkers are elevated (i.e. BNP and troponins) (Daniels and Bayes-Genis 2014). In the Pro-BNP Investigation of Dyspnea in the Emergency department (PRIDE) study, ST2 levels were increased in patients hospitalized for HF, and higher ST2 values were correlated with increased risk of death (Januzzi et al. 2007). Similarly to BNP and troponins, serial ST2 measurements during HF hospitalization were predictive of mortality (Boisot et al. 2008; Anand and Rector 2014). In several studies (Table 1), ST2 has demonstrated some advantages over BNP. There is less variation in sST2 levels relative to age, gender, BMI and renal function, and there is less intra-individual variation in ST2 levels over time (Daniels and Bayes-Genis 2014). Additionally, ST2 is the strongest predictor of mortality both in acute and chronic HF compared to all other biomarkers (Gaggin et al. 2014; Bayes-Genis et al. 2014). Because ST2 is an indicator of cardiac remodelling and fibrosis, antihypertrophic therapies with beta-blockers (Gaggin et al. 2013) and mineralocorticoid receptor antagonists (COACH Trial) (Maisel et al. 2014) should be initiated and maximized in HF patients with elevated ST2 levels.
Procalcitonin
In the critically ill patient, it is often difficult to determine if symptoms of the systemic inflammatory response are due to underlying infection or other aetiologies, and few early markers of infection have proved reliable. Procalcitonin (PCT) may be a useful marker of bacterial infection. Although the exact mechanism of PCT production is unknown, serum levels of the 116 amino acid peptide are increased in the setting of bacterial infection and sepsis (Schwarz et al. 2000; Assicot et al. 1993; Muller et al. 2000). Increased PCT levels can help differentiate between bacterial and viral infections (Gendrel et al. 1998) or between bacterial infection and disease flair of autoimmune disorders (Moosig et al. 1998). Additionally, procalcitonin levels were shown to be normalised in patients with bacterial infection as they were treated with antibiotics (Assicot et al. 1993).
Clinical Use of Procalcitonin in Heart Failure
In patients with a history of CHF presenting with dyspnoea, it is often initially unclear if respiratory symptoms are due to pulmonary oedema or underlying infection. Although many studies suggest PCT can be useful at distinguishing bacterial infection from heart failure exacerbation (Table 1), one study, however, did demonstrate that CHF alone could increase PCT levels independently from infection. It was thought to be due to increased endotoxin resorption in the small bowel in volume-overloaded patients, which can cause falsely elevated PCT in the serum, although patients with HF and underlying bacterial infections were found to have the highest PCT levels (Wang et al. 2014). Despite this study’s results, there remains compelling evidence that in patients with acute heart failure exacerbation and elevated PCT levels, treatment with antibiotics results in better outcomes.
Abbreviations
ACS acute coronary syndrome
ADHF acute decompensated heart failure
BNP Brain or B-Type natriuretic peptide
cTnT cardiac troponin T
ED emergency department
HF heart failure
hsTnT high-sensitivity troponin T
NP natriuretic peptides
NPR natriuretic peptide receptors
NT-proBNP N-terminal pro b-type natriuretic peptide
References:
Ambrosy AP, Fonarow GC, Butler J et al. (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol, 63(12): 1123-33.
PubMed ↗
Anand IS, Fisher LD, Chiang YT et al. (2003) Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation, 107(9): 1278-83.
PubMed ↗
Anand IS, Rector TS (2014) Pathogenesis of anemia in heart failure. Circ Heart Fail, 7(5): 699-700. PubMed ↗
Assicot M, Gendrel D, Carsin H et al. (1993) High serum procalcitonin concentrations in patients with sepsis and infection. Lancet, 341(8844): 515-8.
PubMed ↗
Bayes-Genis A, de Antonio M, Vila J et al. (2014) Head-to-head comparison of 2 myocardial fibrosis biomarkers for long-term heart failure risk stratification: ST2 versus galectin-3. J Am Coll Cardiol, 63(2): 158-66.
PubMed ↗
Berger R, Huelsman M, Strecker K et al. (2002) B-type natriuretic peptide predicts sudden death in patients with chronic heart failure. Circulation, 105(20): 2392-7.
PubMed ↗
Bertrand ME, Simoons ML, Fox KA et al. (2000) Management of acute coronary syndromes: acute coronary syndromes without persistent ST segment elevation; recommendations of the Task Force of the European Society of Cardiology. Eur Heart J, 21(17): 1406-32.
PubMed ↗
Bettencourt P, Ferreira S, Azevedo A et al. (2002) Preliminary data on the potential usefulness of B-type natriuretic peptide levels in predicting outcome after hospital discharge in patients with heart failure. Am J Med, 113(3): 215-9.
PubMed ↗
Bodor GS, Porterfield D, Voss EM et al. (1995) Cardiac troponin-I is not expressed in fetal and healthy or diseased adult human skeletal muscle tissue. Clin Chem, 41(12 Pt 1): 1710-5.
PubMed ↗
Boisot S, Beede J, Isakson S et al. (2008) Serial sampling of ST2 predicts 90-day mortality following destabilized heart failure. J Card Fail, 14(9): 732-8.
PubMed ↗
Braunwald E, Antman EM, Beasley JW et al. (2000) ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina). Circulation, 102(10): 1193-209.
PubMed ↗
Cheng V, Kazanagra R, Garcia A et al. (2001) A rapid bedside test for B-type peptide predicts treatment outcomes in patients admitted for decompensated heart failure: a pilot study. J Am Coll Cardiol, 37(2):386-91.
Collinson PO, Boa FG, Gaze DC (2001) Measurement of cardiac troponins. Ann Clin Biochem, 38(Pt 5): 423-49.
PubMed ↗
Costello-Boerrigter LC, Boerrigter G, Redfield MM et al. (2006) Amino-terminal pro-
B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol,47(2): 345-53.
PubMed ↗
Daniels LB, Bayes-Genis A (2014) Using ST2 in cardiovascular patients: a review. Future Cardiol, 10(4): 525-39.
PubMed ↗
Daniels LB, Clopton P, Bhalla V et al. (2006) How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am Heart J, 151(5): 999-1005.
PubMed ↗
Daniels LB, Maisel AS (2007) Natriuretic peptides. J Am Coll Cardiol, 50(25) 2357-68.
PubMed ↗
Das SR, Drazner MH, Dries DL et al. (2005) Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation, 112(14): 2163-8.
PubMed ↗
Dokainish H, Zoghbi WA, Lakkis NM et al. (2005) Incremental predictive power of B-type natriuretic peptide and tissue Doppler echocardiography in the prognosis of patients with congestive heart failure. J Am Coll Cardiol, 45(8): 1223-6.
PubMed ↗
Doust JA, Pietrzak E, Dobson A et al. (2005) How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ, 330(7492): 625.
PubMed ↗
Felker GM, Hasselblad V, Tang WH et al. (2012) Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail, 14(11): 1257-64.
PubMed ↗
Gaggin HK, Motiwala S, Bhardwaj A et al. (2013) Soluble concentrations of the interleukin receptor family member ST2 and beta-blocker therapy in chronic heart failure. Circ Heart Fail, 6(6): 1206-13. PubMed ↗
Gaggin HK, Szymonifka J, Bhardwaj A, Belcher A et al. (2014) Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail, 2(1): 65-72.
PubMed ↗
Gendrel D, Raymond J, Assicot M et al. (1998) [Procalcitonin, C-reactive protein and interleukin 6 in bacterial and viral meningitis in children]. [Article in French] Presse Med. 1998;27(23): 1135-9.
PubMed ↗
Go AS, Mozaffarian D, Roger VL et al. (2014) Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation, 129(3): e28-e292. J Am Coll Cardiol
PubMed ↗
.
Januzzi JL Jr, Camargo CA, Anwaruddin S et al. (2005) The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol, 95(8): 948-54.
PubMed ↗
Januzzi JL Jr, Peacock WF, Maisel AS et al. (2007) Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol, 50(7): 607-13.
PubMed ↗
Kazanegra R, Cheng V, Garcia A et al. (2001) A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: a pilot study. J Card Fail, 7(1): 21-9.
PubMed ↗
Kieser A, Goodnight J, Kölch W et al. (1995) Identification of the primary growth response gene, ST2/T1, as a gene whose expression is differentially regulated by different protein kinase C isozymes. FEBS Lett, 372(2-3): 189-93.
PubMed ↗
Kojima M, Minamino N, Kangawa K et al. (1989). Cloning and sequence analysis of cDNA encoding a precursor for rat brain natriuretic peptide. Biochem Biophys Res Commun, 159(3): 1420-6.
PubMed ↗
Kuball J, Schmitz FW, Voss RH et al. (2005) Cooperation of human tumor-reactive CD4+ and CD8+ T cells after redirection of their specificity by a high-affinity p53A2.1-specific TCR. Immunity, 22(1): 117-29. PubMed ↗
Latini R, Masson S, Anand IS et al. (2007) Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation, 116(11): 1242-9.
PubMed ↗
Lloyd-Jones D, Adams R, Carnethon M et al. (2009) Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation, 119(3): e21-181.
PubMed ↗
Logeart D, Thabut G, Jourdain P et al. (2004) Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol, 43(4): 635-41. PubMed ↗
Maisel A (2006) The coming of age of natriuretic peptides: the emperor does have clothes! J Am Coll Cardiol, 2006;47(1): 61-4.
PubMed ↗
Maisel A, Hollander JE, Guss D et al. (2004) Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT). A multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. J Am Coll Cardiol, 44(6): 1328-33.
PubMed ↗
Maisel A, Mueller C, Adams K Jr. et al. (2008) State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail, 10(9): 824-39.
PubMed ↗
Maisel A, Xue Y, Greene SJ et al. (2015) The potential role of natriuretic peptide-guided management for patients hospitalized for heart failure. J Card Fail, 21(3): 233-9.
Maisel A, Xue Y, van Veldhuisen DJ et al. Effect of spironolactone on 30-day death and heart failure rehospitalization (from the COACH Study). Am J Cardiol, 114(10): 737-42.
PubMed ↗
Maisel AS, Krishnaswamy P, Nowak RM et al. (2002) Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med, 347(3): 161-7.
PubMed ↗
McCullough PA, Duc P, Omland T et al. (2003) B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis, 41(3): 571-9.
PubMed ↗
Mehra MR, Uber PA, Park MH et al. (2004) Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol, 43(9): 1590-5.
Moe GW, Howlett J, Januzzi JL et al (2007) N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation, 115(24): 3103-10.
Moosig F, Csernok E, Reinhold-Keller E et al. (1998) Elevated procalcitonin levels in active Wegener's granulomatosis. J Rheumatol, 25(8): 1531-3.
PubMed ↗
Mozaffarian D, Benjamin EJ, Go AS et al. (2015) Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation, 131(4): e29-322.
PubMed ↗
Mueller C, Scholer A, Laule-Kilian K et al. (2004) Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med, 350(7): 647-54.
PubMed ↗
Muller B, Becker KL, Schachinger H et al. (2000) Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med, 28(4): 977-83.
PubMed ↗
Nakagawa O, Ogawa Y, Itoh H et al. (1995) Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an "emergency" cardiac hormone against ventricular overload. J Clin Invest, 96(3): 1280-7.
PubMed ↗
Nambi V, Liu X, Chambless LE et al. (2013) Troponin T and N-terminal pro-B-type natriuretic peptide: a biomarker approach to predict heart failure risk--the atherosclerosis risk in communities study. Clin Chem. 2013;59(12): 1802-10.
PubMed ↗
Parmacek MS, Solaro RJ (2004) Biology of the troponin complex in cardiac myocytes. Prog Cardiovasc Dis, 47(3): 159-76.
PubMed ↗
Pascual-Figal DA, Casas T, Ordonez-Llanos J et al. (2012) Highly sensitive troponin T for risk stratification of acutely destabilized heart failure. Am Heart J, 163(6): 1002-10.
PubMed ↗
Peacock WF 4th, De Marco T, Fonarow GC et al. (2008) Cardiac troponin and outcome in acute heart failure. N Engl J Med, 358(20): 2117-26.
PubMed ↗
Redfield MM, Rodeheffer RJ, Jacobsen SJ et al. (2002) Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol, 40(5): 976-82.
PubMed ↗
Ricchiuti V, Voss EM, Ney A et al. (1998) Cardiac troponin T isoforms expressed in renal diseased skeletal muscle will not cause false-positive results by the second generation cardiac troponin T assay by Boehringer Mannheim. Clin Chem, 44(9): 1919-24.
PubMed ↗
Sanada S, Hakuno D, Higgins LJ et al. (2007) IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest, 117(6): 1538-49.
PubMed ↗
Sarzani R, Dessi-Fulgheri P, Paci VM et al. (1996) Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest, 19(9): 581-5.
PubMed ↗
Saunders JT, Nambi V, de Lemos JA et al. (2011) Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation, 123(13):1367-76.
PubMed ↗
Schmitz J, Owyang A, Oldham E et al. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity, 23(5): 479-90. PubMed ↗
Schwarz S, Bertram M, Schwab S et al. (2000) Serum procalcitonin levels in bacterial and abacterial meningitis. Crit Care Med, 28(6): 1828-32.
PubMed ↗
Sharma S, Jackson PG, Makan J. Cardiac troponins. J Clin Pathol, 57(10): 1025-6.
PubMed ↗
Sudoh T, Maekawa K, Kojima M et al. (1989) Cloning and sequence analysis of cDNA encoding a precursor for human brain natriuretic peptide. Biochem Biophys Res Commun, 159(3): 1427-34. PubMed ↗
Tominaga S (1989) A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett, 258(2): 301-4.
PubMed ↗
Tsutamoto T, Wada A, Sakai H et al. (2006) Relationship between renal function and plasma brain natriuretic peptide in patients with heart failure. J Am Coll Cardiol,47(3): 582-6.
PubMed ↗
Wang TJ, Larson MG, Levy D et al. (2002) Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol, 90(3): 254-8.
PubMed ↗
Wang TJ, Larson MG, Levy D et al. (2004) Impact of obesity on plasma natriuretic peptide levels. Circulation, 109(5): 594-600.
PubMed ↗
Wang W, Zhang X, Ge N et al. (2014) Procalcitonin testing for diagnosis and short-term prognosis in bacterial infection complicated by congestive heart failure: a multicenter analysis of 4,698 cases. Crit Care, 18(1): R4.
PubMed ↗
Weir RA, Miller AM, Murphy GE et al. (2010) Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol, 55(3): 243-50. PubMed ↗
Wettersten N, Maisel AS (2016) Biomarkers for heart failure: an update for practitioners of internal medicine. Am J Med, 129(6): 560-7.
PubMed ↗
Xue Y, Clopton P, Peacock WF et al. (2011) Serial changes in high-sensitive troponin I predict outcome in patients with decompensated heart failure. Eur J Heart Fail, 13(1):37-42.
PubMed ↗
Yanagisawa K, Tsukamoto T, Takagi T et al. (1992) Murine ST2 gene is a member of the primary response gene family induced by growth factors. FEBS Lett, 302(1): 51-3.
PubMed ↗
Yancy CW, Jessup M, Bozkurt B et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol, 62(16): e147-239.
PubMed ↗
Yasue H, Yoshimura M, Sumida H et al. (1994) Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation, 90(1): 195-203.
PubMed ↗
Yoshimura M, Yasue H, Okumura K et al. (1993) Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation, 87(2): 464-9.
PubMed ↗








