ICU Management & Practice, Volume 22 - Issue 3, 2022
A Practical Approach for Intensivists
A practical approach to analgesia, sedation and neuromuscular blockade of critically ill patients and a discussion on potential benefits, adverse effects and current professional international recommendations.Introduction
Patients hospitalised in the Intensive Care Unit (ICU) are naturally prone to experience pain. They may require administration of sedatives and even neuromuscular blockade (NMB) in some cases. At the present moment, there are several clinical practice guidelines in this regard by multiple professional associations. However, there are still some discrepancies on the optimal clinical approach of these patients, namely on drug selection alongside monitoring of their effects. In this paper, we introduce a practical approach to the analgesia, sedation and NMB of critically ill patients, whilst taking into account potential benefits, adverse effects and current professional international recommendations.
Evaluation and Management of Pain in the ICU
More than 50% of critically ill patients experience pain, a situation that is associated with adverse outcomes. These include increasing length of ICU stay and in-hospital stay, increasing days under invasive mechanical ventilation (IMV), and a higher incidence of delirium. Clinicians must implement strategies for the early detection, evaluation and management of pain, in an attempt to maximise patient comfort, since this is considered an essential part of the so-called Humanisation of Intensive Care Units.
The physiological response to pain commonly presents with tachycardia, hypertension, tachypnoea, respiratory alkalosis, among others. This response is related to haemodynamic instability, impairment of the immune system and hyperglycaemia, in addition to the release of catecholamines, cortisol and vasopressin. Persistence of pain predisposes to a wide variety of detrimental psychological effects including agitation, post-traumatic stress disorder, disorientation and depression.
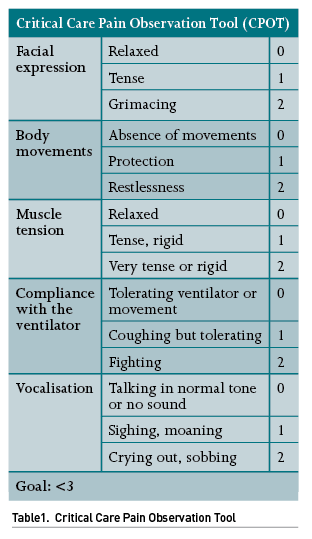
The first step of this approach is to accurately identify pain, which may pose a challenge in patients in which verbal communication is not feasible, for instance, in patients under IMV or sedatives, as well as in patients with paralysis, neurological or neuromuscular disorders, among others. The most widely used scale for pain detection and assessment is the Critical-Care Pain Observation Tool (CPOT) (Table 1), which takes into consideration a handful of clinical parameters; of note, this scale can be used in patients who can verbally communicate and also in patients who cannot, such as those under IMV, as it considers facial expression, upper limb movements and compliance with mechanical ventilation.
Once pain is identified, an adequate analgesic treatment must be prompted. The drugs most frequently used for pain management in the ICU include acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs) and opioids. Other drugs, such as ketamine, lidocaine, neuromodulators and magnesium sulfate, may also be used. Another strategy to be considered is that of regional analgesia, more widely used in post-surgical patients, which will not be addressed in this review.
Indications for therapeutic or prophylactic administration of pain medications in the ICU include the following:
- Patients with endotracheal intubation and IMV
- Polytrauma
- Burns
- Post-operative period
- During procedures such as tracheostomy, placement of pleural tubes, dressing and debridement of wounds, drainage of fluid collections, catheter placements, etc.
- Chronic pain (e.g., cancer)
- Neuropathic pain
- Palliative care
Opioids are considered first-choice drugs for analgesia in critically ill patients due to their high efficacy. These act on the µ, κ and δ receptors of the central nervous system (CNS). The most highly recommended of them are remifentanil, fentanyl, morphine and hydromorphone given their higher analgesic effectiveness. Other less advised opioid medications include buprenorphine, oxycodone, nalbuphine and codeine, which are associated with a higher incidence of adverse effects and a lower analgesic potency.
Opioid accumulation is associated with the following side effects: nausea, vomiting, ileus, haemodynamic instability and respiratory depression. Therefore, their use should be restricted to short periods of time. It is highly encouraged to use the minimum effective dose to achieve the desired effect, since higher doses might cause tolerance and desensitisation of receptors, thereby reducing their effect and, in turn, further requiring higher doses. To minimise adverse effects, a multimodal analgesia with adjuvant drugs may be used, with the aim of blocking the transmission of pain by other mechanisms at the peripheral level and at the level of the spinal cord-hypothalamus-cerebral cortex axis.
Opioid-induced hyperalgesia results from their use for prolonged periods of time along with excessive doses (Lee 2011). It is originated by an impaired action from N-methyl-D-aspartate (NMDA) glutamatergic receptors and an increase in spinal dynorphin levels, resulting in an excessive synthesis and a release of excitatory neuropeptides, thus shifting the balance between antinociceptive and pronociceptive systems. For the above reasons, opioids should be withdrawn as early as possible - as soon as the cause of the pain is solved.
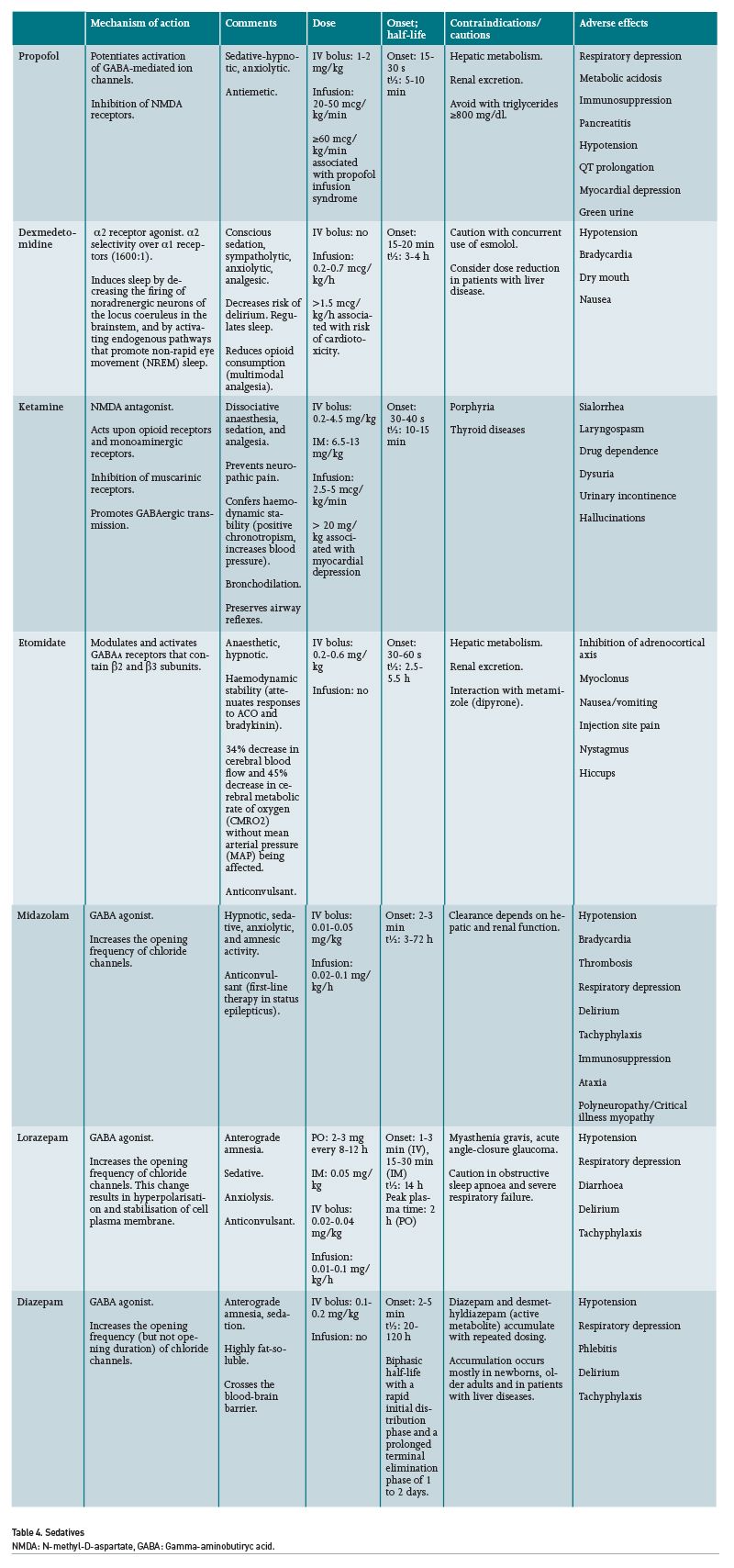
As a result of its ultra-short action and its elimination by plasmatic esterases, remifentanil is the opioid medication of choice. This drug is associated with lower days under IMV, lower time to extubation and lower length of ICU stay. Its pharmacokinetics are not affected by renal or hepatic impairment; therefore, it is safe in patients with liver or kidney diseases. Its major disadvantages include its high cost and lower availability compared to other opioid medications (Yang 2021). Fentanyl is associated with more days of IMV compared to remifentanil. Morphine is associated with hypotension due to histamine release, pruritus, and a higher incidence of nausea and vomiting. Along with hydromorphone, these drugs are reasonable options for analgesia (Devlin 2018).
Acetaminophen is recommended as an adjuvant analgesic in the opioid therapy of critically ill patients. Dose adjustment should be considered in chronic liver failure, and this drug must be avoided in acute liver failure and in cases of allergy. Nefopam is a histamine H1 receptor antagonist focused on the inhibition of monoamine uptake in synapses, which would lead to an increase in noradrenaline, dopamine and serotonin. It is advised as an adjuvant treatment to opioids and as a treatment alternative. However, it is seldom available worldwide.
NSAIDs remain an adequate alternative for analgesia. Their effect is comparable to low-potency opioids, thereby reducing opioid consumption, as well as their side effects. There is a wide variety of drugs included in this group such as COX-1, COX-2 and prostaglandin E2 inhibitors. Among critically ill patients, their adverse effects include acute kidney injury and gastrointestinal (GI) bleeding, with even higher risks for patients with pre-existing impaired renal blood flow, older adults, patients with heart disease, and patients with shock or those exposed to other nephrotoxic drugs (Thadhani 1996). Other lower incidence deleterious effects include cardiovascular and cerebrovascular complications, fluid retention, hypertension and thromboembolic events. In particular, ketorolac has been associated with a significant increase in the incidence of anastomotic leaks in post-operative patients (Wick 2017). However, NSAIDs are not recommended for routine use in critically ill patients.
In subanaesthetic doses, ketamine exerts an analgesic effect comparable to morphine, with a similar need for rescue doses. This drug reduces chronic hyperalgesia mediated by NMDA receptors, as well as that induced by opioid medications (Hirota 2011). Its advantages include the fact that it does not cause respiratory or haemodynamic depression, hence it is useful in patients with shock (Eikermann 2012). Its adverse effects are dose-dependent, and they include hypersalivation, nausea and vomiting, vivid dreams, blurry vision, hallucinations, nightmares and delirium. Due to its dissociative effects, ketamine proves useful in the pain management of severely burned patients or in those with a large number of invasive devices and procedures. It can also be safely used in patients with intracranial hypertension. Ketamine is metabolised via the liver and excreted by the kidneys, nevertheless, no significant adverse effects over hepatic and renal functions have been noted at subanaesthetic doses.
Sedation
Although maintenance sedation is a commonly used approach in critically ill patients, it is intrinsically harmful. This intervention is associated with patient weakness, delirium, increasing days on mechanical ventilation and increasing days of hospitalisation and ICU stay (Nedergaard 2022). However, it might be necessary in some specific scenarios. Indications for maintenance sedation include the following (Reade 2014):
- Moderate to severe acute respiratory distress syndrome (ARDS).
- Intracranial hypertension (e.g., severe traumatic brain injury with concurrent mass effect).
- Status epilepticus (when non-responsive to first-line or second-line therapy).
- Consider in abdominal compartment syndrome, flail chest and patients requiring major surgery, or inability to perform regional anaesthesia.
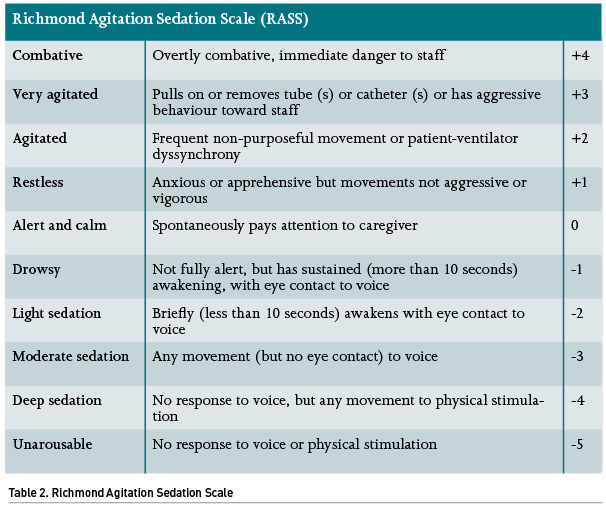
If opting for sedation, it is highly recommended that the dose of the medications is titrated according to predefined goals based on the patient’s condition, as well as continuous monitoring. There are several ways to monitor the sedation state of a critically ill patient; the RASS scale is the most widespread tool for this purpose. If a given patient has an indication for deep sedation (e.g., ARDS or refractory intracranial hypertension) it is recommended that they remain at a level <-3. However, if only minimal sedation is planned (such as in a patient under a weaning protocol), it is reasonable to remain in levels from 0 to -1. It is difficult to find a justification for maintaining a moderate sedation (RASS 2 to 3). There are different technological sedation monitors such as the unilateral or bilateral bispectral index (BIS), entropy monitoring, among others, although none has been shown superior to the RASS scale (Table 2), and they could indeed prompt additional expenses.
Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU (PADIS) guidelines endorse propofol and dexmedetomidine as first-choice sedatives. Benzodiazepines are not recommended as maintenance sedatives due to their association with delirium (Devlin 2018). Ketamine might also be considered as a sedative for critically ill patients, even showing benefits in patients with shock including lower rates of hypotension and bradycardia (Umunna 2015).
The haemodynamic changes associated with propofol may include myocardial depression, bradycardia and hypotension. It must be taken into consideration that propofol provides up to 1.1 kcal per millilitre; therefore, it may cause hypertriglyceridaemia, pancreatitis or overfeeding. With regard to dexmedetomidine, this drug frequently causes bradycardia, and it may also contribute to hypotension in patients with shock; nevertheless, its safety profile appears to be better than other sedatives and it has even been associated with greater haemodynamic stability in patients with septic shock. Unfortunately, despite many recommendations discouraging the use of benzodiazepines, midazolam remains the most commonly used sedative in continuous sedation among many hospitals (Luz 2022). It is recommended to consider the use of anti-psychotic agents, volatile anaesthetics or intermittent benzodiazepines in patients with ARDS who do not achieve deep sedation with propofol and dexmedetomidine.
It is important to emphasise that the condition that leads the patient to require sedation must be solved as soon as possible, and a wake-up test must be performed early in the course to prompt a timely ICU discharge, which should be repeated every day until the patient can be safely withdrawn from mechanical ventilation. This procedure is associated with fewer days on IMV, and fewer days of ICU stay.
Neuromuscular Blockade
Neuromuscular blockade (NMB) can be common among critically ill patients, especially in the course of ARDS treatment. Its indications are limited, and this modality is associated with several adverse effects such as venous thromboembolism, critical illness myopathy, patient awareness during paralysis, autonomic interactions, pressure ulcers, corneal ulcers and residual paralysis (Renew 2020).
Indications of NMB in the ICU include:
- Rapid sequence intubation (RSI)
- Moderate to severe ARDS
- Consider in intracranial hypertension, refractory status asthmaticus, failed sedation, and to temporarily reduce intra-abdominal pressure in patients with intra-abdominal hypertension (IAH), among others (De Laet 2007).
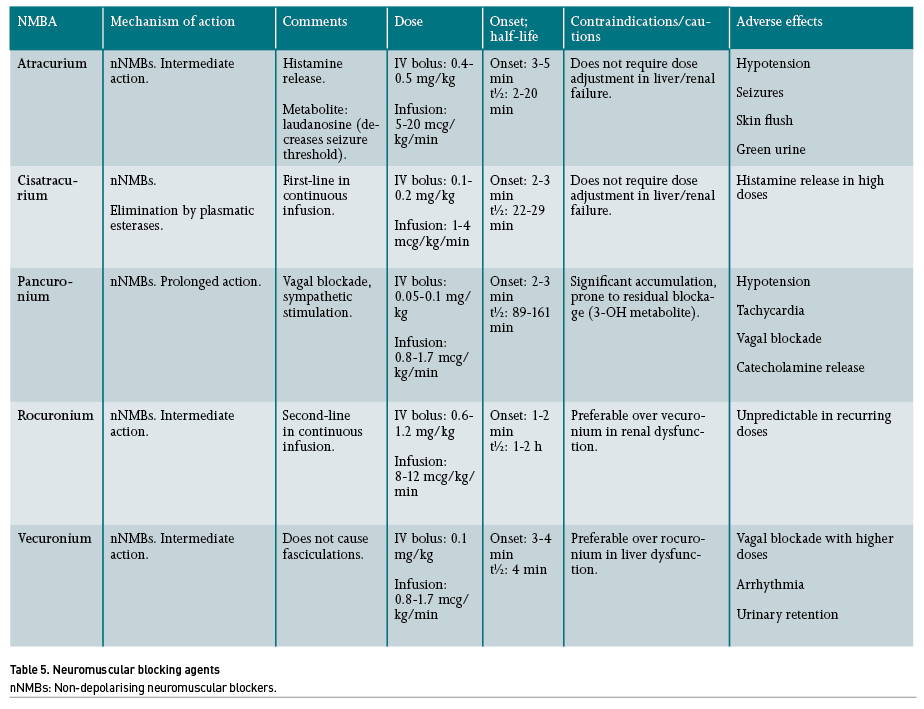
The American Society of Anesthesiologists (ASA) recommends the use of NMB to reduce the number of intubation attempts, thereby decreasing the risk of airway injuries during direct laryngoscopy (Apfelbaum 2013). Rocuronium is the only non-depolarising drug indicated for induction and intubation during RSI.
Regarding the management of moderate to severe ARDS in patients under IMV, meta-analyses have shown a reduction in mortality in the ICU when using an infusion of cisatracurium (Ho 2020), and recently, it has shown greater utility if maintained under continuous infusion for more than 48 hours in patients with respiratory failure under IMV due to COVID-19 (Li 2021). The major advantage of cisatracurium relies on its metabolism by Hofmann elimination, which confers a rapid elimination of its effects when withdrawn. Moreover, it does not depend on hepatic or renal depuration. A strategy that combines the use of NMB with cisatracurium and low tidal volume could reduce mortality, most likely due to a reduction of asynchrony events and improvement of pulmonary compliance and functional residual capacity, which would translate into an increase in oxygenation (Murray 2016; Battaglini 2021; Chang 2020).
Among neurocritical patients with acute brain injury, the use of NMB has been suggested in order to reduce the number of episodes of intracranial hypertension; however, the level of evidence for this recommendation is low (Renew 2020). Its main effect relies on the reduction of asynchrony events with the ventilator, cough limitation and any other condition that may cause Valsalva manoeuvre (Steingrub 2014). In patients with intra-abdominal hypertension and abdominal compartment syndrome, NMB has also been suggested in order to increase abdominal compliance by relaxation of the abdominal muscles (Malbrain 2005). Nonetheless, conclusive evidence in this regard is lacking.
There are other neuromuscular blocking agents (NMBA) not currently recommended as first-choice drugs; however, their use in specific scenarios may be justified when the latter are not available. In patients with ARDS that require NMB, vecuronium, atracurium or pancuronium may also be used, although with the risk of prolonging neuromuscular relaxation, thereby increasing side effects. In addition, when rocuronium is not available for RSI, succinylcholine can also be considered, which is a depolarising NMB of ultra-short action, although with the risk of hyperkalaemia and even malignant hyperthermia (Zamarrón-López 2019).
Train-of-four nerve stimulation (TOF) is a tool for the monitoring of NMB. A value <0.7 is considered an adequate paralysis (Murphy 2010). In a recent study that compared three strategies for using NMB (a fixed-dose of cisatracurium; titration based solely on TOF and a ventilator synchrony protocol), it was shown that a protocol using ventilator synchrony for cisatracurium titration required significantly less drug compared to TOF-based titration and a fixed dosing regimen (DiBridge 2021).
Several factors affect the duration of NMBA activity: for instance, the concomitant use of diuretics, antiarrhythmics, aminoglycosides, magnesium, lithium, as well as some conditions such as hypokalaemia, hypothermia and acidosis, all increase the potency of non-depolarising NMBA. NMBA potency is inversely related to its speed of onset (that is, the lower the potency of the drug, the faster the onset of neuromuscular blockade after its administration). Patients with myasthenia gravis and glaucoma are especially sensitive to the effects of NMB. On the other hand, patients with burns are resistant to the effects of NMBA due to the proliferation (upregulation) of nicotinic receptors in the sarcolemma (Murray 2016).

Conclusions
Analgesics, sedatives and neuromuscular blocking agents are commonly used medications in the ICU. An adequate protocol of care involves knowledge of their indications, adverse effects, their correct use and the selection of the most appropriate agent, with the aim of reducing morbimortality in critically ill patients.
Conflict of Interest
None.
References:
Adams DR, Tollinche LE, Yeoh CB et al. (2020) Short-term safety and effectiveness of sugammadex for surgical patients with end-stage renal disease: a two-centre retrospective study. Anaesthesia. 75(3):348-352.
Anderson BJ (2008) Paracetamol (Acetaminophen): mechanisms of action. Paediatr Anaesth. (10):915-21.
Apfelbaum JL, Hagberg CA, Caplan RA et al. (2013) Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 118(2):251–70.
Barr J, Puntillo K, Ely EW et al. (2013) Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit. Critical Care Medicine. 41(1):263–306.
Battaglini D, Sottano M, Ball L et al. (2021) Ten golden rules for individualized mechanical ventilation in acute respiratory distress syndrome. Journal of Intensive Medicine, 1(1):42-51.
Brinck EC, Tiippana E, Heesen M et al. (2018) Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst Rev. 20;12(12):CD012033.
Chang W, Sun Q, Peng F et al. (2020) Validation of neuromuscular blocking agent use in acute respiratory distress syndrome: a meta-analysis of randomized trials. Crit Care. 24, 54.
Chanques G, Constantin JM, Devlin J.W et al. (2020) Analgesia and sedation in patients with ARDS. Intensive Care Med. 46:2342–2356.
Chanques G, Constantin JM, Devlin JW et al. (2020) Analgesia and sedation in patients with ARDS. Intensive Care Med. 46(12):2342-2356.
Chen W, Liu J, Yang Y, Ai Y et al. (2022) Ketorolac Administration After Colorectal Surgery Increases Anastomotic Leak Rate: A Meta-Analysis and Systematic Review. Front Surg. 9;9:652806.
Choi M, Wang L, Coroneos CJ et al. (2021) Managing postoperative pain in adult outpatients: a systematic review and meta-analysis comparing codeine with NSAIDs. CMAJ. 2021 Jun 14;193(24):E895-E905.
Cohen SP, Mao J (2014) Neuropathic pain: mechanisms and their clinical implications. BMJ. 348:f7656.
Cohen SP, Bhatia A, Buvanendran A et al. (2018) Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 43(5):521-546.
De Laet I, Hoste E, Verholen E, De Waele JJ (2007) The effect of neuromuscular blockers in patients with intra-abdominal hypertension. Intensive Care Med. 33(10):1811-4.
Devlin JW, Skrobik Y, Gélinas C et al. (2018) Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 46(9):e825-e873.
DiBridge JN, Rivosecchi RM, McVerry BJ et al. (2021) Comparison of three cisatracurium dosing strategies in acute respiratory distress syndrome: A focus on drug utilization and improvement in oxygenation. J Crit Care. 66:166-172.
Dunn LK, Durieux ME (2017) Perioperative Use of Intravenous Lidocaine. Anesthesiology. 126(4):729-737.
Eichenberger U, Neff F, Sveticic G et al. (2008) Chronic phantom limb pain: the effects of calcitonin, ketamine, and their combination on pain and sensory thresholds. Anesth Analg. 106(4):1265-73.
García B, Latorre S, Torre F et al. (2010) Hidromorfona: una alternativa en el tratamiento del dolor, revista de la sociedad española del dolor. 17(3):153-161.
Graham GG, Davies MJ, Day RO et al. (2013) The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 21(3):201-32.
Günther T, Dasgupta P, Mann A et al. (2018) Targeting multiple opioid receptors - improved analgesics with reduced side effects? Br J Pharmacol. 175(14):2857-2868.
Hermanns H, Hollmann MW, Stevens MF et al. (2019) Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 123(3):335-349.
Krasel C, Bünemann M, Lorenz K et al. (2005) Beta-arrestin binding to the beta2-adrenergic receptor requires both receptor phosphorylation and receptor activation. J Biol Chem. 280(10):9528-35.
Kelly SJ, Moran JL, Williams PJ et al. (2016) Haemodynamic effects of parenteral vs. enteral paracetamol in critically ill patients: a randomised controlled trial. Anaesthesia.(10):1153-62.
Lee A, Cooper MG, Craig JC et al. (2007) Effects of nonsteroidal anti-inflammatory drugs on postoperative renal function in adults with normal renal function. Cochrane Database Syst Rev. (2):CD002765.
Legriel S, Oddo M, Brophy GM (2017) What’s new in refractory status epilepticus? Intensive Care Medicine. 43: 543–6.
Li Bassi G, Gibbons. K, Suen JY et al. (2005) Intra-abdominal hypertension in the critically ill: it is time to pay attention. Curr Opin Crit Care. 2005 Apr;11(2):156-71.
Li Bassi et al. (2021) Use of Neuromuscular Blocking Agents in Mechanically Ventilated Patients with COVID-19: A Propensity Score Analysis. European Respiratory Journal. 58.
Lumanauw DD, Youn S, Horeczko T et al. (2019) Subdissociative-dose Ketamine Is Effective for Treating Acute Exacerbations of Chronic Pain. Acad Emerg Med. (9):1044-1051.
Luz M, Brandão B, de Castro REV et al. (2022) Practices in sedation, analgesia, mobilization, delirium, and sleep deprivation in adult intensive care units (SAMDS-ICU): an international survey before and during the COVID-19 pandemic. Ann. Intensive Care 12, 9.
Migdady I., Rosenthal ES, Cock HR (2022) Management of status epilepticus: a narrative review. Anaesthesia, 77(S1), 78–91.
Mizobuchi Y, Miyano K, Manabe S et al. (2022) Ketamine Improves Desensitization of µ-Opioid Receptors Induced by Repeated Treatment with Fentanyl but Not with Morphine. Biomolecules. 10;12(3):426.
Murphy GS, Sorin BJ (2010) Bloqueo neuromuscular residual, Anestesia y analgesia. 111(1):120-128.
Murray MJ, DeBlock H, Erstad B et al. (2016) Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 44(11):2079–103.
Narayanan M, Venkataraju A, Jennings J (2016) Analgesia in intensive care: part 1, BJA Education. 16(2):72–78.
Nedergaard HK, Korkmaz S, Olsen HT et al. (2022) Failure of non-sedation strategy in critically ill, mechanically ventilated patients - a retrospective, post-hoc analysis of the NONSEDA trial. J Crit Care. 68:66-71.
Neme D, Aweke Z, Micho H et al. (2020) Evidence-Based Guideline for Adult Sedation, Pain Assessment, and Analgesia in a Low Resource Setting Intensive Care Unit: Review Article. Int J Gen Med. 13:1445-1452
Ono Y, Tanigawa K, Shinohara K et al. (2016) Difficult airway management resources and capnography use in Japanese intensive care units: a nationwide cross-sectional study. J Anesth. 30(4):644–52.
Ong CK, Seymour RA, Lirk P, Merry AF (2010) Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 110(4):1170-9.
Papazian L, Forel JM, Gacouin A et al. (2010) Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 363(12):1107–16.
Price D, Kenyon NJ, Stollenwerk N (2012). A fresh look at paralytics in the critically ill: real promise and real concern. Annals of intensive care, 2(1), 43.
Raffa RB, Pawasauskas J, Pergolizzi JV Jr et al. (2018) Pharmacokinetics of Oral and Intravenous Paracetamol (Acetaminophen) When Co-Administered with Intravenous Morphine in Healthy Adult Subjects. Clin Drug Investig. 38(3):259-268.
Reade MC, Finfer S (2014) Sedation and delirium in the intensive care unit. N Engl J Med. 370(5):444-454.
Renew JR, Ratzlaff R, Hernández-Torres V et al. (2020) Neuromuscular blockade management in the critically Ill patient. J intensive care. 8, 37.
Roman-Vendrell C, Yu YJ, Yudowski GA (2012) Fast modulation of μ-opioid receptor (MOR) recycling is mediated by receptor agonists. J Biol Chem. 27;287(18):14782-91.
Schwenk ES, Viscusi ER, Buvanendran A et al. (2018) Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 43(5):456-466.
Schmid CL, Kennedy NM, Ross NC et al. (2017) Bias Factor y Therapeutic Window Correlate to Predict Safer Opioid Analgesics. Celúla. 171 :1165–1175.e13.
Shah SB, Chawla R, Pahade A et al. (2021) Neuromuscular blockers and their reversal: have we finally found the on-off switches?. Ain-Shams J Anesthesiol 13, 15.
Sottile PD, Kiser TH, Burnham EL et al. (2018) An Observational Study of the Efficacy of Cisatracurium Compared with Vecuronium in Patients with or at Risk for Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 197(7):897-904.
Sridharan K, Sivaramakrishnan G (2019) Comparison of Fentanyl, Remifentanil, Sufentanil and Alfentanil in Combination with Propofol for General Anesthesia: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr Clin Pharmacol. 14(2):116-124.
Stamer UM, Erlenwein J, Freys SM et al. (2021) Perioperative analgesia with nonopioid analgesics : Joint interdisciplinary consensus-based recommendations of the German Pain Society, the German Society of Anaesthesiology and Intensive Care Medicine and the German Society of Surgery. Chirurg. 92(7):647-663.
Steingrub JS, Lagu T, Rothberg MB et al. (2014) Treatment with neuromuscular blocking agents and the risk of in-hospital mortality among mechanically ventilated patients with severe sepsis. Crit Care Med. 42(1):90-6.
Umunna BP, Tekwani K, Barounis D et al. (2015) Ketamine for continuous sedation of mechanically ventilated patients. J Emerg Trauma Shock. 8(1):11.
Watari K, Nakaya M, Kurose H (2014) Multiple functions of G protein-coupled receptor kinases. J Mol Signal. 6;9(1):1.
Wick EC, Grant MC, Wu CL (2017) Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA Surg. 152(7):691-697.
Zhang J, Barak LS, Winkler KE et al. (1997) A central role for beta-arrestins and clathrin-coated vesicle-mediated endocytosis in beta2-adrenergic receptor resensitization. Differential regulation of receptor resensitization in two distinct cell types. J Biol Chem. 272(43):27005-14.












