ICU Management & Practice, ICU Volume 12 - Issue 3 - Autumn 2012
Authors
Sascha Tafelski, MD
Maxim Kartachov, MD
Claudia Spies, MD
Department of Anaesthesiology and Intensive Care
Charité University Hospital
Campus Charité Mitte and Campus Virchow-Klinikum
Berlin, Germany
Introduction
Debates have long existed on whether gender might either impact intensive care unit (ICU) outcome in general or only in distinct patient populations. Studies have enrolled patients without regard for a balance of gender distribution; hence, results mainly based on male patients have been used for females similarly.
One of the first sets of data that emphasised relevant differences
between genders described patients with acute coronary syndrome. Goldberg et
al. Showed that female patients presented with different symptoms in the
initial phase of acute myocardial infarction (Goldberg et al. 1998).
Furthermore, evidence was provided that showed that discrepancies in initial presentation
led to differences in clinical outcome (Srichaiveth et al. 2007). These and
other results led to the conclusion that there is a strong need to assess
gender effects to avoid possible differences in care processes provided and in
outcomes from therapeutic measures. Consequently, this raised a first question
that only seems to be answered simply.
Is There a Clinical Outcome Difference Between Genders in the ICU?
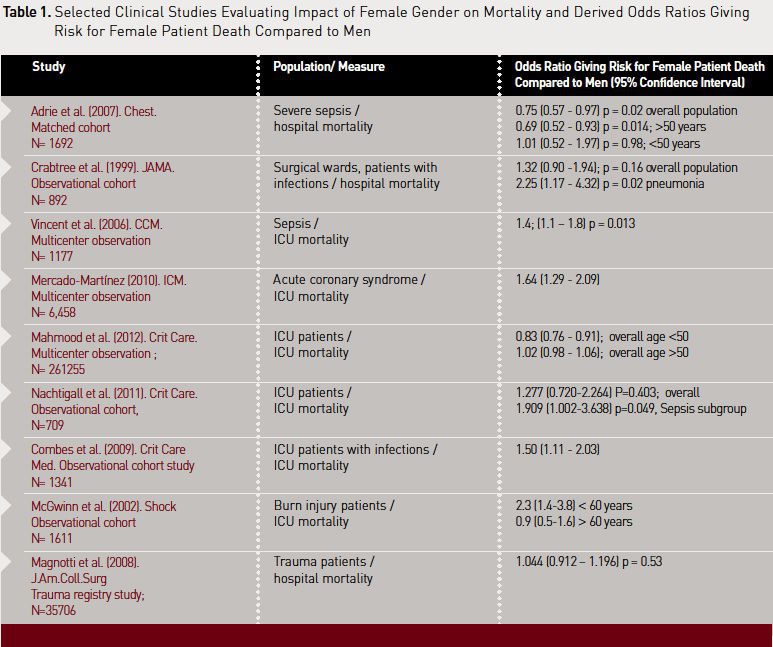
In past years, studies in intensive care have revealed very conflicting results regarding gender-related effects on outcome. Some authors have found female gender to be associated with lower ICU mortality (Ayanian and Epstein 1991; Adrie et al. 2007), some have described equal outcome (Crabtree et al. 1999; Wichmann et al. 2000) and others have shown significant associations between female gender and increased ICU mortality (Seymour et al. 2010; Vincent et al. 2006; Mercado-Martinez et al. 2010).
For the specific subgroup of patients with infections, differences in
outcome and course of sepsis between genders cause equally controversial
discussions. A recent retrospective study from Mahmood and colleagues showed
that in a sample of more than 250,000 ICU patients in the US, female gender was
associated with lower mortality when comparing patients under 50 years of age
with an adjusted odds ratio (OR) of 0.83 (95% confidence interval: 0.76–0.91)
(Mahmood et al. 2012). The subset of patients older than 50 years showed a
different picture, however. Here, no difference in mortality was found
(adjusted OR: 1.02, 95% confidence interval: 0.98–1.06). Interestingly, for
patients admitted with sepsis there was a trend for higher mortality in
females, but this difference was not of a significant level (adjusted OR: 1.07,
95% confidence interval: 0.99–1.16, P=0.08). Unfortunately, no data is provided
for the subset of older females with sepsis as age was found to interact with
results.
Our own prospectively collected
data evaluated 709 mainly elderly patients and demonstrated that gender does
not influence mortality in the main ICU population. However, in the analysis of
the subset of patients with sepsis, female gender was significantly associated
with fatal outcome, with an OR of 1.966 (95% confidence interval: 1.045–3.701,
P=0.036) (Nachtigall et al. 2011). We were able to demonstrate that males were
more likely to suffer from infection during ICU stay but that females had a
relevant risk factor for dying with an infection.Table 1 provides a summary of study findings that show a link between female gender and mortality.
Pathophysiology Traces
One of the hypotheses that tries to explain the underlying pathophysiology is related to sex hormone differences, with etiology immune modulatory effects by female steroid hormones discussed. Depending on the hormone levels, oestrogen can be immune reinforcing or even anti-inflammatory. Oestradiol blocks proinflammatory tumor necrosis factor (TNF), interleukin (IL)-1 and IL-6 and, stimulates inhibitory IL- 4 and IL-10. In healthy conditions, especially at pregnancy level, oestrogen enhances nitric oxide (NO) production by stimulating the expression and activity of different isoforms of nitric oxide synthetase (NOS). In contrast, in the presence of lipopolysaccharide (LPS), oestrogen blocks the inducible NOS and consequently the NO production. Furthermore, oestrogen inhibits the formation of oxygen radicals and apoptosis. On the postmenopausal level, oestradiol, stimulates TNF, interferon (INF)-γ and IL- 1β (Straub 2007).
In animal studies, there seems
to be consistency in the positive effect of oestrogen on sepsis and shock
caused by trauma. In haemorrhagic shock and sepsis, female animals show less
immune suppression, higher IL 3 and IL-1 levels, and better chances of survival
than their male counterparts (Wichmann et al. 2000; Zellweger et al. 1997;
Diodato et al. 2001). This effect correlates with the 17β-oestradiol level in the female cycle (Knoferl
et al. 2001). Ovariectomised animals lose this advantage over male animals, but
this can be recovered by administration of 17β-oestradiol (Knoferl et al. 2001; Jarrar et al. 2000). Oestrogen
substitution can also support the hepatic and cardiac function of male animals
with haemorrhagic shock (Mizushima et al. 2000). Furthermore, a blockade of the
testosterone receptors reestablishes the immune function and attenuates the
liberation of IL-1 and IL-2, thus impeding the induction of sepsis and
enhancing survival (Angele et al. 1997; Mizushima et al. 2000) .
Clinical Data to Support Experimental Results
In clinical studies, experimental results could not be clearly reproduced, and so far no conclusive evidence can be found. Schroder and his team were able to show, in a study on 52 septic patients, that women had a better outcome, lower TNF- α and higher IL-10 level (Schroder et al. 1998). Some years later, Adrie and colleagues examined 1,692 patients with severe sepsis and found that women had a lower rate of central venous catheter use, fewer days on a ventilator, a shorter length of stay on ICU and lower mortality (Adrie et al. 2007).
Offner and associates identified
male gender as one risk factor for postoperative infections (Offner et al.
1999), while, in contrast, Eachempati and colleagues found a higher mortality
in female patients with sepsis in the US (Eachempati et al. 1999). Similar
results were found in a European multi-centre study involving 3,147 patients
with sepsis (Vincent et al. 2006). Combes and his team retrospectively
evaluated more than 1,300 patients with nosocomial infections in a mixed ICU
and found an increased risk of death with excess mortality of five percent and
an adjusted odds ratio (OR) of 1.50 (95% Confidence Interval: 1.11-2.03) for
female gender (Combes et al. 2009). Other studies showed higher mortality for
women with blunt abdominal trauma (Napolitano et al. 2001), burns (McGwin et
al. 2002) and sepsis caused by abdominal infection (McLauchlan et al. 1995).
In opposition, there are studies
showing no correlation between gender and mortality (Oberholzer et al. 2000; Magnotti
et al. 2008; Angus et al. 2001). Based on natural scientific understanding,
there should be an explanation for these contradictory results.
Transferability of Experimental Data
Direct translation from bench to bedside seems difficult. Experimental sepsis is short in comparison with the clinical course of sepsis in humans, and the animals involved are younger without comorbidities. Furthermore, in the experimental setting, the onset of sepsis can be tailored to the hormonal status, so most studies are conducted when oestrogen levels are highest. Besides this, animal studies control the surroundings, possibly biasing the results, and there is no gender- based difference in treatment intensity in animal experiments.
Summarising the principle concerns regarding the transferability of the experimental setting on the one hand and the clinical impact of gender as one variable in the complex clinical setting of ICU care on the other hand, there remain concerns, and another hypothesis has been addressed.
Another Hypothesis to Explain Gender Differences
Another hypothesis is related to a difference in healthcare allocation between genders, based on clinical data. Valentin and colleagues found via a large observational cohort study of ICU patients that men received more invasive procedures like mechanical ventilation, central venous or pulmonary artery catheterisation, catecholamine and kidney replacement therapy (Valentin et al. 2003). Although increased overall mortality in female patients was observed, severity of illness-adjusted mortality rate was not different in this study. Thus, the authors conclude that different therapeutic approaches in male patients are not translated into survival advantages. Another study in an emergency department focused on factors for non-adherence to early goal directed therapy. In this setting, Mikkelsen and associates demonstrated that it was less likely for females to receive this therapy; the probability was further reduced when the physician in charge was also female (Mikkelsen et al. 2010). Han and colleagues observed low adherence rates for lung protective ventilation measures in females, conceivably because of their smaller stature (Han et al. 2011).
To evaluate this allocation
factor we also analysed possible gender-related effects in healthcare
distribution and focused on the quality of infection diagnostics and antibiotic
therapy. For both, in the general postoperative cohort study as well as the
subgroup of patients with sepsis, no relevant difference regarding quality of
care was found (Nachtigall et al. 2011). There seems to be a trend for a more
strict indication of diagnostic procedures with exposure to radiation in
females. This difference might be attributed to the intention to limit possible
risks for the ovules. Of course, possible misallocation of therapeutic measures
should be monitored closely, but currently there is no precise evidence that
any existing slight difference of care for female patients would impact ICU
outcome.
Differences in Physiology
Besides differences in anthropometric indices, female patients show some specific differences compared to males. Women have a different distribution volume than men, meaning an altered effect duration and elimination of medical agents used on ICUs. Examples for this effect are seen in the use of midazolam and vancomycin (Greenblatt et al. 1984; Ducharme et al. 1994).
Other examples of differences in
metabolism can be found regarding the CYP-450 system with clinical relevance.
Females show a faster elimination of methylprednisolone while they are more
sensitive to the drug itself (Lew et al. 1993). A differing sensitivity of
receptors may explain the disparity in the effect of morphine between genders
and why propofol-based general anaesthesia is faster degraded in female
patients (Dahan et al. 1998; Gan et al. 1999). Furthermore, there is evidence
that sex hormones interact with pharmaceuticals, for example, hormoneinduced QT
prolongation, having consequent effects on cardioactive medicine and
potentially causing adverse drug reactions (Rodriguez et al. 2001). In the
field of gender-specific influences on medication, lots of question marks
remain, emphasising the need for balanced cohort studies to assess the
effectiveness and safety of established and new drugs.
Different Physiology; Same Scores?
Facing all those differences in physiology, it does not seem to be reasonable to assess sepsis in both genders with the same measurements, and the use of the same intensive care scoring systems may be questioned. The Acute Physiology And Chronic Health Evaluation (APACHE) and the Sequential Organ Failure Assessment (SOFA) methods integrate laboratory and physiological items regardless of the specific limits in both genders, whereas the score performance should be different. Using the same scoring items could lead to a bias concerning the severity of disease, and sepsis might be misstratified. Summarising this, it might be desirable to adopt gender-specific scores and to use gender-related corrections like the Framingham-score (Wilson et al. 1998) or the CHA2DS2-VASc-score in cardiology (Lip et al. 2010).
Conclusion
Gender has been shown to be outcome relevant in the ICU setting. Through consequent considerations from clinical studies, gender is one relevant factor for future evidence-based decision making. Currently, gender related impacts of medical interventions and drug therapy undergo intensive evaluation, and the results of these studies will influence therapy recommendations in future, even gender-specific guidelines could be on the horizon. It is expected that a more detailed understanding of differences in clinical presentation, course of diseases and cure processes will support a better knowledge of underlying pathophysiology.





