ICU Management & Practice, Volume 20 - Issue 4, 2020
Post-ICU patients are at high-risk of clinical deterioration. Continuous and mobile monitoring on hospital wards is useful to detect clinical deterioration at an early stage. It may help to prevent serious adverse events and ICU readmission.
Most patients discharged from ICUs are old and have co-morbidities. Even when they are young and were healthy before ICU admission, the days or weeks spent in the ICU increase the risk of complications such as nosocomial infections, pulmonary atelectasis, airway obstruction, metabolic disturbances, cardiac arrhythmia, thromboembolic events and delirium. Therefore, post-ICU patients are at high risk of clinical deterioration and ICU readmission (Frost et al. 2009). As intensivists, we see the far too common and often frustrating middle of the night preventable ICU readmission. We also recount sad stories of patients who spent weeks recovering from life-threatening conditions and who unexpectedly died hours or days after ICU discharge.
When patients leave the ICU (or the step-down unit) they usually transition from a care area with a high nurse/patient ratio where they are closely monitored to a general ward where nurses have to deal with a large number of patients and often spot check vital signs only every 4-8 hours. The intermittent nature of vital signs monitoring explains that nurses may miss up to 90% of hypoxaemic events (Sun et al. 2015) and 50% of hypotensive events occurring in the wards (Turan et al. 2019). In this setting, continuous monitoring of vital signs has been shown to be associated with a decrease in calls for rescue interventions and cardiac arrest, in ICU transfer and in hospital mortality (Taenzer et al. 2010; Bellomo et al. 2012; Brown et al. 2014; Subbe et al. 2017). However, until recently, monitoring systems were bulky, expensive and designed for the OR and the ICU. Solutions are emerging to upgrade and simplify the way we monitor patients on hospital wards (Vincent et al. 2018, Michard and Sessler 2018; Khanna et al. 2019). They include contact free sensors and wireless wearables, as well as triage tools to help operationalise the use of these. At the same time, rigorous validation and interventional outcome studies are needed as we attempt to bring about a culture change and improve patient safety (Saugel et al. 2020).
Contact Free Sensors (or “Stay-in-Bed” Monitoring Solutions)
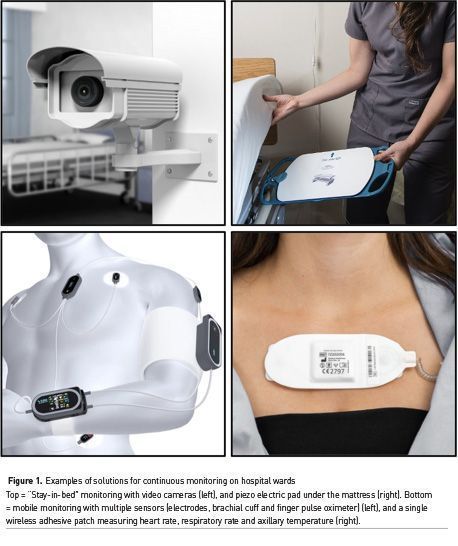
Contact free sensors are either bed sensors or video cameras (Figure 1). Bed sensors are piezo-electric pads to be put under any bed mattress or part of modern high-end hospital beds (Zimlichman et al. 2012). They basically feel heart beats and respiratory movements. They have been used in several clinical studies, including an outcome study showing a reduction in calls for cardiac arrest and in hospital length of stay after implementation (Brown et al. 2014). Video cameras can track subtle changes in colour face to count heart beats and in chest or abdominal movements to detect respiratory rates. Several attempts have also been made to estimate oxygen saturation and blood pressure from video monitoring (Luo et al. 2019), but validation studies done in real hospital conditions are lacking. Contact free solutions are very appealing for patients because they do not need to be connected to any device nor to wear any sensor. However, they do not follow patients when they leave their bed (e.g. to go to the bathroom) or their room (e.g. for physiotherapy). As a matter of fact, we expect most patients to leave their beds as soon and as often as possible to prevent thrombotic complications and bedsores. In surgical patients, early mobilisation is a key element of enhanced recovery (aka ERAS) programs. Therefore, wireless and wearable sensors are highly desirable to make continuous monitoring a reality for a large number of patients who need and wish to move out of their hospital bed as often as possible.
Wireless Wearable Sensors (or Mobile Monitoring Solutions)
Several mobile monitoring systems have been developed and/or validated over the last few years (Michard et al. 2019a; Khanna et al. 2019). They include necklaces, finger sensors and adhesive patches. The first monitoring system specifically developed for the wards comprises a finger sensor to measure oxygen saturation and pulse rate, a wireless brachial cuff for intermittent blood pressure measurements and chest electrodes for the detection of heart beats and the calculation of the pulse wave transit time (PWTT) (Figure 1). The PWTT is the time difference between the peak of the R wave on the ECG (ventricular contraction) and the arrival of the plethysmographic pulse in periphery (finger sensor). The PWTT depends on blood flow and vascular tone so that any change in PWTT indicates either a change in cardiac output or a change in vascular tone. From there, and the knowledge of baseline BP, it becomes possible to estimate BP or, more realistically, to predict a change in BP and hence trigger a brachial cuff measurement. This system has been shown to be useful to decrease the number of rapid response team calls (Weller et al. 2018). The combination of a wireless brachial cuff to measure BP, abdominal adhesive patch to capture RR and finger pulse oximeter to monitor SpO2 and pulse rate has been used with success in a large UK study where continuous ward monitoring was associated with a significant decrease in cardiac arrests and mortality (Subbe et al. 2017). Other “all-in-one” solutions have been developed. They include necklaces, finger devices, and adhesive patches integrating accelerometers, photoplethysmographic, piezo electric and/or bioimpedance sensors. They have the advantage to enable the simultaneous monitoring of several vital signs from a single device (Figure 1).
Who Should Be Monitored?
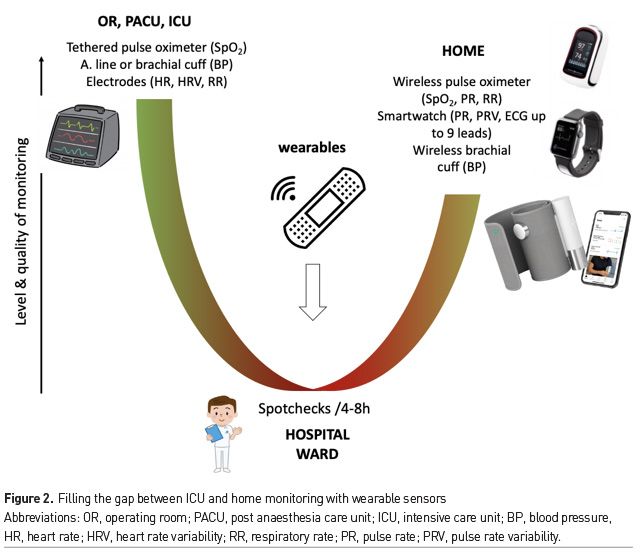
We now live in a world where smartphones and smartwatches can be used to monitor pulse rate, to detect cardiac arrhythmia, to measure oxygen saturation and to record up to 9 ECG leads (Michard et al. 2019b). Soon they may also be able to detect changes in BP, if not measuring it. Therefore, given what is becoming possible from home, one may argue that all inpatients should benefit from close, if not continuous, monitoring (Figure 2). However, old habits die hard and hospital resources are limited so that the transition from intermittent spot-checks of vital signs towards continuous monitoring for all inpatients will likely take time. A first necessary step could be the identification of patients at high risk of clinical deterioration. This subset of inpatients could be identified using simple scores, such as a classic Early Warning Score (EWS) or the new PRODIGY score in patients receiving opioids (Khanna et al. 2020). These scores are easy to calculate but their sensitivity and specificity to predict adverse events remain limited. Once enough clinical and biological data have been collected in the electronic medical record (EMR) hospital system, predictive analytics may help to better identify patients at high risk of deterioration. For instance, the Safety Index, which is a smart fusion of five vital signs has been shown to predict cardiorespiratory deterioration hours before it becomes overt (Hravnak et al. 2011). The eCART, which is a machine learning-derived fusion of age, vital signs and lab data, has been shown to be the best predictor of cardiac arrest, ICU transfer and deaths when compared to the national EWS, the modified EWS and any individual vital sign (Churpek et al. 2016). In other words, predictive analytics has potential to improve the prediction of clinical deterioration and rationalise the use of continuous monitoring techniques on the wards (Michard and Teboul 2019).
What Should We Monitor?
All vital signs have potential value to detect clinical deterioration, but their respective value logically depends on the adverse event to be detected. For instance, in patients with cardiac disease or metabolic disorders (e.g. dyskalaemia), at high risk of cardiac arrhythmia, monitoring heart rate and heart rate variability may be the priority. In contrast, in patients hospitalised for the new coronavirus disease 2019 (COVID-19), who are susceptible to develop ARDS, monitoring RR and SpO2 seems to be a minimum, and enables the calculation of the ROX index (Hill and Ruthazer 2019). Both RR and SpO2 can be monitored with a pulse oximeter, which may also be useful to quantify the respiratory swings in the pulse oximetry waveform (aka pulsus paradoxus), an easy-to-measure marker of respiratory effort (Michard and Shelley 2020). In non-cardiac surgical patients, a nationwide study (Michard et al. 2015) including >200,000 patients from >500 US hospitals showed that most common postoperative adverse events are respiratory and infectious complications, emphasising the importance of monitoring respiratory variables (RR and SpO2) and temperature in this context. Evidence also suggests a strong association of postoperative hypotension and myocardial injury after non-cardiac surgery, and since this insult is devoid of clinical signs and symptoms, continuous monitoring of blood pressure is currently being investigated in pragmatic cluster randomised trials (clinicaltrials.gov/ct2/show/NCT04574908?term=khanna&cntry=US&state=US%3ANC&draw=2&rank=1). During post-operative patient-controlled analgesia (PCA) with opioids, rates of bradypnoea (RR < 10) lasting >3 min can reach 41% (Overdyk et al. 2007). Therefore, monitoring RR becomes the priority.
In the general medical and surgical ward population, studies have repeatedly ranked RR, HR and systolic BP as the top three variables to be monitored. In a study including >250,000 patients and using machine learning methods for predicting clinical deterioration in ward patients (Churpek et al. 2016), RR had the highest “weight” in the predictive algorithm followed by HR, systolic BP, temperature and SpO2. In line with these observations, the National Institute for Health and Care Excellence in the UK stated that “RR is the best marker of a sick patient and is the first observation that will indicate a problem or deterioration in condition” (www.nice.org.uk/guidance/CG50). Anyway, most sensors are now able to measure several vital signs at the same time and the combination (aggregation or fusion) of several variables logically improves the ability to detect clinical deterioration at an early stage and may enable future algorithms to recognise specific patterns and suggest a diagnosis (Michard et al. 2020).
Conclusion
Most patients discharged from the ICU are at high risk of clinical deterioration and may benefit from close monitoring until they fully recover. There is today a gap between ICU monitoring and home monitoring options that could be closed by upgrading monitoring strategies on hospital wards (Figure 2). Emerging solutions to improve and simplify patient monitoring on hospital wards include contact free sensors and wireless wearables. Both enable the early detection of clinical deterioration and may help to prevent severe adverse events such as cardiac arrest and ICU readmission. Wireless wearables give patients the freedom to leave their bed and increase their physical activity, which is useful to enhance clinical recovery, decrease hospital length of stay and improve patient satisfaction.
Conflict of Interest
FM is the founder and managing director of MiCo, a Swiss consulting and research firm. AKK consults for Edwards Lifesciences, Medtronic, Philips North America and Zoll Medical. AK is on the clinical advisory board for Retia Medical and is a founding member for BrainX LLC, a collaborative platform for research and development of AI products in healthcare. He is also funded with a Clinical and Translational Science Institute (CTSI) NIH/NCTAS KL2 TR001421 award for a trial on continuous postoperative haemodynamic and saturation monitoring.
References:
Bellomo R, Ackerman M, Bailey M et al. (2012) A controlled trial of electronic automated advisory vital signs monitoring in general hospital wards. Crit Care Med, 40: 2349–61.
Churpek MM, Yuen TC, Winslow C, et al. (2016) Multicenter comparison of machine learning methods and conventional regression for predicting clinical deterioration on the wards. Crit Care Med, 44:368-374.
Frost SA, Alexandrou E, Bogdanovski T, et al. (2009) Severity of illness and risk of readmission to intensive care: a meta-analysis. Resuscitation, 80:505-10.
Hill NS, Ruthazer R (2019) Predicting outcomes of high-flow nasal cannula or acute respiratory distress syndrome. An index that ROX. Am J Respir Crit Care Med 199:1300-02.
Hravnak M, Devita MA, Clontz A, et al. (2011) Cardiorespiratory instability before and after implementing an integrated monitoring system. Crit Care Med, 39 :65-72.
Khanna AK, Hoppe P, Saugel B (2019) Automated continuous noninvasive ward monitoring: future directions and challenges. Crit Care 23:194.
Khanna AK, Bergese SD, Jungquist CR (2020) Prediction of opioid induced respiratory depression on inpatient wards using continuous capnography and oximetry: an international prospective, observational trial. Anesth Analg 131:1012-1024.
Luo H, Yang D, Barszczyk A, et al. (2019) Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ Cardiovasc Imaging 2019; 12:e008857.
Michard F, Mountford WK, Krukas MR, et al. (2015) Potential return on investment for implementation of perioperative goal-directed fluid therapy in major surgery: a nationwide database study. Perioper Med (Lond), 4:11.
Michard F, Sessler DI (2018) Ward monitoring 3.0. Br J Anaesth, 121:999-1001.
Michard F, Teboul JL (2019) Predictive analytics: beyond the buzz. Ann Intensive Care, 9:46.
Michard F, Bellomo R, Taenzer A (2019a) The rise of ward monitoring: opportunities and challenges for critical care specialists. Intensive Care Med, 45:671-73.
Michard F, Barrachina B, Schoettker P (2019b) Is your smartphone the future of physiologic monitoring? Intensive Care Med, 45:869-71.
Michard F, Shelley K (2020) Should we monitor pulsus paradoxus via pulse oximetry in patients with COVID-19 and acute respiratory failure? Am J Respir Crit Care Med, 202:770-1.
Michard F, Saugel B, Vallet B (2020) Rethinking the post-COVID-19 pandemic hospital: more ICU beds or smart monitoring on the wards? Intensive Care Med, 46:1792-3.
Overdyk FJ, Carter R, Maddox RR, et al. (2007) Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth Analg, 105:412-8.
Saugel B, Hoppe P, Khanna AK (2020) Automated continuous noninvasive ward monitoring: validation of measurement systems is the real challenge. Anesthesiology, 137:402-10.
Subbe CP, Duller B, Bellomo R (2017) Effect of an automated notification system for deteriorating ward patients on clinical outcomes. Crit Care, 21:52.
Sun Z, Sessler DI, Dalton JE, et al. (2015) Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg, 2015; 121:709-715.
Taenzer AH, Pyke JB, McGrath SP, et al. (2010) Impact of pulse oximetry surveillance on rescue events and intensive care unit transfers: A before-and-after concurrence study. Anesthesiology, 112:282-287.
Turan A, Chang C, Cohen B, et al. (2019) Incidence, severity, and detection of blood pressure perturbations after abdominal surgery: a prospective blinded observational study. Anesthesiology, 130:550-9.
Vincent JL, Einav S, Pearse R, et al. (2018) Improving detection of patient deterioration in the general hospital ward environment. Eur J Anaesthesiol, 35:325-333.
Weller RS, Foard KL, Harwood TN (2018) Evaluation of a wireless, portable, wearable multi-parameter vital signs monitor in hospitalized neurological and neurosurgical patients. J Clin Monit Comput, 32:945- 51.
Zimlichman E, Szyper-Kravitz M, Shinar Z, et al. (2012) Early recognition of acutely deteriorating patients in non-intensive care units: assessment of an innovative monitoring technology. J Hosp Med, 7:628-33.