ICU Management & Practice, Volume 16 - Issue 1, 2016
This review explores current definitions of frailty, methods
available to diagnose it, and its application to perioperative and critically
ill patients.
Frailty is increasingly recognised as a potential
contributor to patient outcome during an episode of critical illness. However,
there is currently no consensus definition or assessment tool. Two main models
exist to conceptualise frailty —the “frailty phenotype” and the “deficit
model”. Both models have been validated in the community setting to be predictive
of patient outcomes. However, they are limited in critical care by their
applicability at the bedside. In the community, a diagnosis of frailty is
associated with increased risk of falls, hospitalisation, institutionalisation
and death. The direct pathophysiological pathway that results in a frail state
is currently unknown, despite considerable research in this area. Frail
patients have disordered homeostasis of many systems, including the
inflammatory, coagulation, and neuro-endocrinal systems. The prevalence of frailty
is approximately 1 in 5 elderly patients, and is increased in patients
undergoing major surgery. Frail surgical patients are at higher risk of morbidity
and mortality than non-frail patients. The role of frailty in the critical care
environment is less clear. Thus far, the small number of studies has produced
conflicting results. It appears that frailty has a higher prevalence than in
thecommunity and may be associated with poorer ICU outcomes. However, further
research intothe application of frailty assessment tools in the ICU is
required.
Recently there has been increased recognition of the
importance of functional status for patient outcome following Intensive Care
Unit (ICU) admission. This includes patient functional status both upon ICU
admission and discharge. Similarly, frailty is one determinant of a patient’s functional
state and its impact upon outcomes is being increasingly recognised (McDermid
et al. 2011; McDermid and Bagshaw 2014). Previous literature on this topic has
focused on geriatric and perioperative patients, in whom frailty has been shown
to be associated with an increased risk of hospital admission, institutionalisation,
postoperative complications and mortality (Song et al. 2010; Graham et al.
2009; Fried et al. 2001; Woods et al. 2005; Rockwood et al. 2005; Xue 2011;
Partridge et al. 2012). However, the lack of a consensus definition and
uncertainty on how to best assess frailty have resulted in limited research in
the critically ill. There is increasing evidence suggesting that the
consideration of frailty in critically ill patients may provide additional
prognostic information.
What is Frailty?
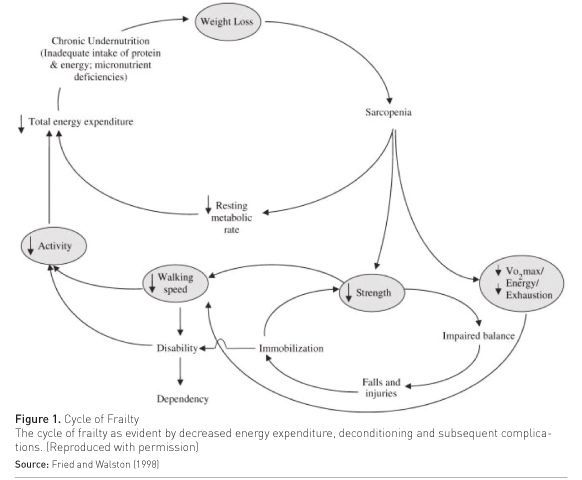
There is currently no consensus definition of frailty nor a single definitive assessment tool, despite recent international attempts at consensus (Fried et al. 2001; Xue 2011). However, frailty is commonly conceptualised as a “ multidimensional geriatric syndrome characterised by an increased vulnerability, resulting from an ageassociated decline in reserve and function, such that the ability to cope with everyday or acute stressors is compromised” (Xue 2011). The precise pathophysiology of frailty is incompletely understood. Frailty appears to be differentiated from normal ageing by the accumulation of multiple pathological abnormalities that contribute to frailty’s characteristic clinical manifestations of sarcopenia, malnutrition, and decreased energy expenditure (Hubbard and Woodhouse 2010) (Figure 1).
The primary pathological process has been postulated to be chronic inflammation
(Hubbard and Woodhouse 2010). Other significant pathological contributors are
likely to include disordered coagulation, as well as dysfunction of the immune
and neuro-endocrinal systems (Hubbard et al. 2009a; Waltson et al. 2002; Hunt et
al. 2010). These suppositions are supported by research revealing that frail
patients have significantly altered levels of C-reactive protein, interleukin-6,
tumour necrosis-factor, as well as elevated clotting factor VIII, fibrinogen
and D-dimer levels. Additionally, frail patients are more likely to have a
disordered hypothalamicpituitary axis, disordered glucose metabolism and lower
vitamin D levels (Hunt et al. 2010; Bayliss et al. 2013; Ensrud et al. 2011;
Leng et al. 2004).
How these biological abnormalities contribute to frailty is
also poorly understood. It is known that there exists a strong association
between chronically elevated inflammatory cytokines and decreased physical
performance, muscle weakness, atrophy, and the progression of disability in
elderly patients (Cesari et al. 2004; Ferruci et al. 1999). However, elderly
patients often have altered biochemical markers as a result of other concurrent
chronic illnesses such as renal failure and cardiovascular disease (Waltson et
al. 2002).
Thus it appears that a critical mass of abnormalities in a
patient’s homeostatic systems is required before a frail state develops
(Hubbard and Woodhouse 2010). Further confounding current pathophysiological
models is the find- ing that these observed biological abnormalities might not
entirely account for the pathology underlying a frail state. The psychosocial
environment of a patient may also impact on the genesis of frailty. This is
supported by research demonstrating frailty’s association with smoking, lower
socio-economic status and lack of physical exercise (Hubbard et al. 2009b; Lang
et al. 2009).
How to Diagnose and Measure Frailty
To date, several methods have been developed to diagnose and
measure frailty (Whitson et al. 2007). A recent review identified 27 published tools
for the assessment of frailty that range from isolated biochemical
abnormalities or physiological parameters to detailed multidisciplinary team assessments
(Bouillon et al. 2013; Sternberg et al. 2011). However, frailty research and debate
is driven by two distinct but validated frailty models. The first model views
frailty as a physical syndrome or ‘phenotype’. The second model views frailty
as a collection of deficits in measurable health domains, (the ‘deficit’ model)
(Sternberg et al. 2011). A recent review revealed that 83% of published
literature on frailty used either of these models (Bouillon et al. 2013).
Phenotype Model
The phenotype model is based on the pioneering work of Fried
et al. in North America. Fried et al. followed more than 5300 elderly patients
for seven years, and found the presence of greater than three of the following
features: unintentional weight loss, weakness, low energy levels, slowness, and
decreased physical activity characterized a frail state (Fried et al. 2001). In
this study a frail state was predictive of falls, hospitalisation, and death
with adjusted hazard ratios of 1.29 (CI 1-1.68), 1.29 (CI 1.09-1.54), and 2.24
(CI 1.51-3.33) at 3 years respectively. This was the first study to show that,
although age and co-morbidity were associated with frailty, they did not define
frailty itself and frailty exists as a distinct clinical entity. Additionally
this study was the first to assess patients in the community, as opposed to
patients already admitted to hospital or other healthcare institutions.
Frailty Model
The alternative approach considers frailty as the accumulation
of numerous health deficits. The greater the number of deficits a patient
acquires, the higher risk of a frail state (Mitnitski et al. 2001; Rockwood and
Mitnitski 2011). The Canadian Health Study of Aging is the largest study
utilising this approach. This study looked at over 10,000 elderly patients, and
after integrating patient co-morbidities, clinical examinations findings and an
assessment of activities of daily living, developed a 70-point frailty index. The
frailty index was shown to be predictive of death and institutiona lisation at
70 months, with hazard ratios of 1.26 (CI 1.24–1.29) and 1.56 (CI 1.48–1.65),
respectively (Rockwood et al. 2007). It may be contended that the frailty index
approach is consistent with the concept that development of frailty is a
gradual process rather than an absent or present phenomenon. Rockwood et al.
have suggested that the frailty index may be a more robust and sensitive measure
of frailty-associated outcomes than the frailty phenotype (Rockwood et al.
2007). However, Bouillon et al. have suggested that the two models of frailty
have similar predictive and discriminative ability to detect frailty (Bouillon et
al. 2013).
Limitations
A major limitation of many frailty assessment tools is their
impracticality in many clinical contexts. Many tools are not suitable for use
at the bedside, time-consuming to perform, require specially trained
clinicians, or the performance of specialised biochemical and physical
investigations. Tools Newer tools have been developed that may overcome some of
these barriers and increase their practicality, especially in critical care.
• Clinical Frailty Scale: The Clinical Frailty Scale (CFS) has been derived from the frailty index developed in the Canadian Health Study of Aging. The CFS is a simple visual analogue 9-point scale ranging from very fit (1) to terminally ill (9), with a score greater than 4 indicating frailty (Figure 2). In a cohort of over 2,300 patients, the CFS was shown to be predictive of six-month mortality and institutionalisation, with hazard ratios of 1.30 (CI 1.27–1.33) and 1.46 (CI 1.39–1.53) respectively. Furthermore, it has been validated against the frailty index showing a high degree of correlation, with a Pearson correlation coefficient of 0.80, p < 0.01 (Rockwood et al. 2005). The greatest benefit of the CFS is its ease of use, lack of required ancillary testing, and its ability to be applied at the bedside.

• Edmonton Frailty
Scale: Another simple but validated tool is the Edmonton Frailty Scale (EFS).
The EFS incorporates a brief 10-point assessment of specific health domains,
including cognition, medication usage, nutrition and social supports.
Functional status is assessed via the ‘timed up and go’ or TUG test. The EFS
has been validated for use by primary care physicians and geriatricians (Hilmer
et al. 2009), and also has been shown to be predictive of outcome in acute general
medical in-patients and patients admitted with acute coronary syndromes (Hilmer
et al. 2009; Graham et al. 2013). Rolfson et al. (2006) demonstrated the ease of
EFS usage in 158 elderly patients. Using a non-medical assessor and a trained
geriatrician to assess frailty, Rolfson et al. found a high level of agreement
between the EFS values obtained by both assessors (Pearson correlation
coefficient 0.64, p < 0.01) and importantly that the EFS took less than 5 minutes
to administer (Rolfson et al. 2006). Although a simple and validated tool, the EFS
is potentially limited in its assessment in the critically ill by the need for
a practical functional assessment.
Tool Comparison
To date, there has been limited comparison between the
different frailty tools to predict patient outcomes. A systematic review by De
Vries et al. concluded that whilst many tools have construct validity,
comparisons between them are limited and difficult due to the range of
population groups assessed. The various combinations of clinical and investigation
parameters used further limit comparison (de Vries et al. 2011). A 2008
editorial contended that different tools to assess frailty may be required in
different situations and no one tool may be ideal, e.g. bedside clinician versus
public health officers seeking to explore population-based trends and planning
requirements (Martin and Brighton 2008).
Frailty in the Community
The prevalence of frailty in the community has been reported
to be between 6.9% to 22.7%, depending on the population studied and the tool
used (Collard et al. 2012). Frailty has been consistently shown to have a
higher prevalence in women and increasing prevalence with advancing age (Song
et al. 2010; Graham et al. 2009; Woods et al. 2005; Collard et al. 2012). Furthermore,
it appears that it is more common in Southern Europe and South America, perhaps
reflecting cultural differences in diagnosing frailty in these respective
populations (Santos-Eggimann et al. 2009; Alvarado et al. 2008).
Frailty in the Perioperative Setting
Frailty is a significant predictor of outcome in elderly
patients presenting for surgery, regardless of the frailty assessment tools
employed (Partridge et al. 2012). The reported prevalence of perioperative
frailty varies markedly between 4-50% (Makary et al. 2010; Sepeheri et al.
2014), but is generally higher than that in community-based studies,
irrespective of the surgery type. This may be influenced by the underlying
indication for surgery, as the common surgical indications of cardiovascular or
malignant diseases are also associated with a frail state.
Current evidence suggests that frailty is associated with
significant postoperative complications. In mixed general, vascular and
orthopaedic surgical populations frailty has been associated with increased
delirium, infection, thromboembolic disease and pressures areas (Partridge et
al. 2012). It has also been associated with prolonged hospital length of stay
and an increased risk of institutionalisation post discharge (Partridge et al.
2012). One study reported that one-third of patients aged >65 years assessed
as frail pre-operatively were institutionalised at 6 months post major elective
surgery (Robinson et al. 2011).
Frailty has a potentially significant influence upon
outcomes in the cardiac surgery and transplantation populations. Frailty,
assessed in a variety of ways and as a component of a comprehensive perioperative
assessment, has been shown to be associated with increased morbidity and mortality
following cardiac surgery (Sepeheri et al. 2014; Afilalo et al. 2010; Lee et
al. 2010; Sundermann et al. 2011). One study revealed that frailty was a more
reliable predictor of oneand twelve-month mortality when compared to the more
commonly administered EuroScore (Sundermann et al. 2011). In addition, the
Fried frailty phenotype has been shown to be a better predictor of both quality
of life and mortality in liver transplantation candidates than the traditional
Model for End-Stage Liver Disease (MELD) score (Derck et al. 2015; Lai et al.
2014).The high prevalence of frailty in perioperative patients and its
association with adverse postoperative outcomes may offer a unique opportunity for
multidisciplinary-focused patient assessment and postoperative planning.
Frailty and Critical Care
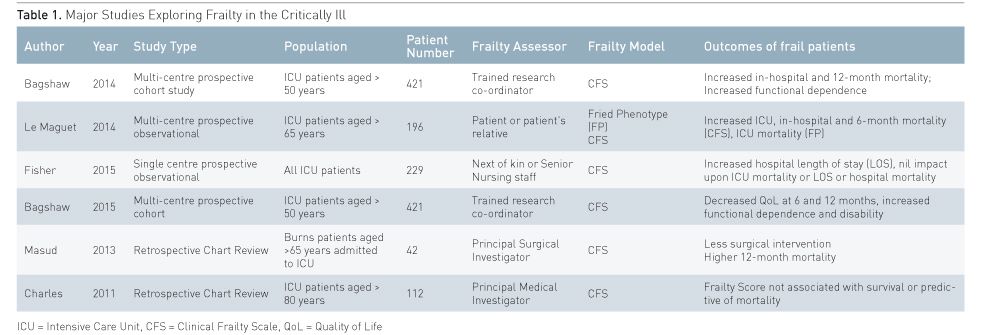
Frailty is increasingly recognised as a potential contributor to critically ill patient outcome. However, research has been limited by difficulty using frailty diagnostic tools, due to a lack of premorbid history, the presence of interceding acute critical illness and the impracticality of applying many of the tools at the bedside. Thus, existing evidence of the utility of frailty assessment in critically ill patients is derived from a relatively small number of studies (Table 1). Bagshaw et al. conducted a multicentre study in Canadian Intensive Care Units’ (ICU) looking at frailty in 421 patients. Bagshaw utilised trained assessors to apply the CFS to all patients aged over 50 years of age at the time of ICU admission. They found that frailty was present in one in three patients and was associated with an increased risk of both hospital, adjusted odds ratio 1.81, (CI 1.09– 3.01) and 12-month mortality, adjusted hazard ratio 1.82, (CI 1.28–2.60), when compared to non-frail patients (Bagshaw et al. 2014). A follow-up study indicated that frail patients had significantly lower quality of health scores at 6 and 12 months (Bagshaw et al. 2015). A major limitation of this study was that only one in three of all eligible patients was included, questioning the practical application of frailty assessment in critically ill patients.
In France Le Maguet et al. performed a multicentre observational
study of 196 patients aged greater than 65 years to investigate frailty, and employed
both the Fried frailty phenotype and the CFS. This study permitted either the
patient or their relatives to provide the necessary information to assign a
frailty score. Frailty was found to be present in 41% and 23% of patients,
using the Fried phenotype and the CFS, respectively. In this study, frailty, as
defined by Fried’s phenotype, was three times more likely to be associated with
ICU mortality. In addition a CFS >4 was significantly associated with
hospital and 6-month mortality (Le Maguet et al. 2014). However, the high number
of patients with traumatic brain injury (20%) or admitted post cardiac arrest
(8%) potentially confounded and reduced the generalisability of these findings.
Other research has focused on more specific sub-groups of
critically ill patients with the CFS. Masud et al. assessed the correlation of
frailty with outcome in elderly patients with severe burns. They found that
frailty was associated with less surgery and higher mortality at 12 months (Masud
et al. 2013).
Unfortunately, the correlation of frailty and adverse
outcomes is not consistent in studies of the critically ill. In a retrospective
assessment of over 100 patients aged greater than 80 years from the United
Kingdom, Charles et al. (2011) found no correlation between the frailty score and
adverse patient outcomes. Fisher et al. (2015) utilised the CFS in a single
centre study of over 200 patients in an Australian tertiary hospital. They found
that frailty, as defined by the CFS > 4 had a prevalence of 13%, was more
common in chronic liver and chronic renal disease patients, and was significantly
associated with increased hospital length of stay but not ICU or hospital
mortality. In contrast to other studies Fisher et al. applied the CFS to all
patients admitted to the ICU regardless of age and used a patient’s next of
kin, or senior nursing staff when next of kin were unavailable, to assign the
CFS score. Consistent with other studies of frailty in the critically ill, only
half of all eligible patients were able to be included in the study (Fisher et
al. 2015).
Whilst current ICU predictive tools use age and co-morbidities
in their models, they incorporate a very limited assessment of patients’
pre-morbid function. Interestingly, within both Bagshaw's and Le Maguet’s
studies there appeared to be no significant difference in ICU illness severity
scoring between frail and non-frail patients. This suggests that the assessment
of frailty may potentially be an adjunct to existing predictive tools in
quantifying a patient’s pre-morbid reserve and post ICU discharge outcome.
Further confounding the role of frailty in the critically
ill has been the suggestion that an episode of critical illness may rapidly
accelerate a patient’s pre-frail state or lead to the development of many of
the characteristics of frailty. Baldwin et al. explored this in patients with
respiratory failure who required ICU admission. Frailty was assessed
immediately prior to hospital discharge via the Fried phenotype model. This
study found frail patients had a 6-month mortality of 41%, and that with each
increased Fried phenotype domain mortality increased three-fold (Baldwin et al.
2014). The application of frailty scoring at discharge may allow greater
quantification of the physical, nutritional, cognitive and psychological disabilities
of ICU survivors. This in turn may allow directed interventions to minimise
long lasting sequelae.
Choice of Assessor
The ideal person needed to assess frailty in critical care
is currently unknown. In the community, geriatricians have formal training and
expertise in recognising and managing frail patients and accordingly are shown
to have high inter-rater reliability (Rockwood et al. 2005; Rockwood et al.
2007). However, it is unclear whether this consistency exists outside this
setting. In the studies performed in the critically ill, a variety of assessors
have been used and there is no published data assessing their inter-rater
reliability. Bagshaw et al. (2014;2015) utilised trained assessors to assign
scores. Le Maguet (2014) and Fisher et al. (2015) utilised the patient’s next
of kin or, in their absence, a senior ICU nurse. Interestingly, in the latter
study there was no statistical difference between the frailty scores assigned
by these two methods. The problem of variability in inter-rater reliability was
highlighted by Hii et al., who found that non-geriatrician clinicians were
unable to accurately diagnose frailty and varied significantly in classifying
frailty (Hii et al. 2015).
Conclusions
Frailty may be an important factor in predicting patient
outcome in ICU and is being increasingly studied in different patient
populations. Prevalence is approximately 1 in 5 of elderly patients in the
community and is even higher in those undergoing major surgery or experiencing critical
illness. Although evidence for a strong association between frailty and outcome
exists in the community, current evidence suggests an inconsistent association
between frailty and adverse patient outcomes in the critically ill. In addition,
it remains unclear which tool is most appropriate to use for the assessment of
frailty in ICU and who should be making such assessments. Further research is
required into the assessment of frailty in the critically ill before its
routine use can be recommended for prediction of outcome after ICU admission.
References:
Afilalo J, Eisenberg M, Morin J et al. (2010) Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol, 56 (20): 1668-76.
Alvarado BE, Zunzunegui MV, Beland F (2008) Life course social and health conditions linked to frailty in Latin American older men and women. J Gerontol A Biol Sci Med Sci, 63(12): 1399–1406.
Bagshaw SM, Thomas HT, McDermid RC et al. (2014) Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ, 186(2): E95-102.
Bagshaw S, Stelfox H, Johnson J et al. (2015) Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med, 43(5): 973-82.
Baldwin MR, Reid MC, Westlake AA (2014) The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. J Crit Care, 29(3): 401-8.
Bayliss D, Bartlett D, Syddall H et al. (2013) Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Dordr), 35(3): 963–71.
Bouillon K, Kivimaki M, Hamer M et al. (2013) Measures of frailty in population-based studies: an overview. BMC Geriatr, 21;13:64.
Cesari M, Penninx B, Laurentani F et al. (2004) Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci, 59(3): 242-8.
Charles B, Porter R, Bryden D (2011) Feasibility and utility of frailty assessment in the over 80’s on critical care. Crit Care, 15(Supp 1): P499.
Collard R, Boter H, Schoevers R et al. (2012) Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. J Am Geriatr Soc, 60(8): 1487–92.
Derck J, Thelen A, Cron D et al. (2015) Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation, 99(2): 340-4.
de Vries N, Staal J, Van Ravensburg C et al. (2011) Outcome instruments to measure frailty: a systematic review. Ageing Res Rev, 10(1): 104-14.
Ensrud K, Blackwell T, Cauley J et al. (2011) Circulating 25-hydroxyvitamin D levels and frailty in older men: the osteoporotic fractures in men study. J Am Geriatr Soc, 59 (1): 101-6.
Ferruci L, Harris T, Guralnik J et al. (1999) Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc, 47(6): 639-46.
Fisher C, Karalapillai D, Bailey M et al. (2015) Predicting intensive care and hospital outcome with the Dalhousie Clinical Frailty Scale: a pilot assessment. Anaesth Intensive Care, 43(3): 361-8.
Fried L, Tangen C, Walston J et al. (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Med Sci, 56(3): M146-56.
Fried LP, Walston J. Frailty and failure to thrive. In: Hazzard WR, Blass JP, Ettinger WH Jr, Halter JB, Ouslander J, eds. Principles of geriatric medicine and gerontology. 4th ed. New York: McGraw Hill; 1998:1387–1402.
Graham J, Snih S, Berges I et al. (2009) Frailty and 10-year mortality in community-living Mexican American older adults. Gerontology, 55 (6): 644–51.
Graham M, Galbraith P, O'Neill D et al. (2013) Frailty and outcome in elderly patients with acute coronary syndrome. Can J Cardiol, 29(12): 1610-5.
Hii T, Lainchbury, J Bridgman G (2015) Frailty in Acute Cardiology: Comparison of a Quick Clinical Assessment Against a Validated Frailty Assessment Tool. Heart Lung Circ, 24(6): 551–6.
Hilmer S, Perera V, Mitchell S et al. (2009) The assessment of frailty in older people in acute care. Australas J Ageing, 28(4): 182-8.
Hubbard R, Woodhouse K (2010) Frailty, inflammation and the elderly. Biogerontology, 11(5): 635-41.
Hubbard R, O’Mahony, Savva G, et al (2009a) Inflammation and frailty measures in older people. J Cell Mol Med, 13(9B): 3103-9.
Hubbard R, Searle S, Mitnitski A et al. (2009b) Effect of smoking on the accumulation of deficits, frailty and survival in older adults: a secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging, 13 (5): 468-72.
Hunt K, Walsh B, Voegeli D et al. (2010) Inflammation in aging part 1: physiology and immunological mechanisms. Biol Res Nurs, 11(3): 245–52.
Lai J, Feng S, Terrault N et al. (2014) Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant, 14(8): 1870-9.
Lang I, Hubbard R, Andrew M et al. (2009) Neighborhood deprivation, individual socioeconomic status, and frailty in older adults. J Am Geriatr Soc, 57(10): 1776-80.
Lee D, Buth K, Martin B et al. (2010) Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation, 121(8): 973-8.
Leng S, Cappola A, Andersen R et al. (2004) Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res, 16(2): 153-7.
Le Maguet P, Roquilly A, Lasocki S (2014) Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med, 40(5): 674-82.
Makary MA, Segev DL, Pronovost PJ et al. (2010) Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg, 210(6): 901-8.
Martin FC, Brighton P (2008) Frailty: different tools for different purposes? Age Ageing, 37(2): 129-31.
Masud D, Norton S, Smailes S et al. (2013) The use of a frailty scoring system for burns in the elderly. Burns, 39(1): 30-6.
McDermid RC, Stelfox HT, Bagshaw SM (2011) Frailty in the critically ill: a novel concept. Crit Care, 15(1): 301.
McDermid RC, Bagshaw SM (2014) Scratching the surface: the burden of frailty
in critical care. Intensive Care Med, 40(5): 740-2.
Mitnitski AB, Mogilner AJ, Rockwood K (2001) Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal, 1: 323–36.
Partridge JS, Harai D, Dhesi JK (2012) Frailty in the older surgical patient: a review. Age Ageing, 41(12): 142-7.
Robinson TN, Wallace JI, Wu DS et al. (2011) Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg, 213(1): 37-42.
Rockwood K, Song X, MacKnight C et al. (2005) A global clinical measure of fitness and frailty in elderly people. CMAJ, 173(5): 489-95.
Rockwood K, Mitnitski A (2011) Frailty defined by deficit accumulation
and geriatric medicine defined by frailty. Clin Geriatr Med, 27(1): 17–26.
Rockwood K, Andrew M, Mitnitski A (2007) A comparison of two approaches to measuring frailty in elderly people. J Gerontol Series A Biol Sci Med Sci, 62(7): 738–43.
Rolfson D, Majumdar S, Tsuyuki R et al. (2006) Validity and reliability of the Edmonton Frail Scale. Age Ageing, 5(5): 526-9.
Santos-Eggimann B, Cuénoud P, Spagnoli J et al.(2009) Prevalence of frailty in middle-aged and oldercommunity-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci, 64(6): 675–81.
Sepeheri A, Beggs T, Hassan A et al. (2014) The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg, 148(6): 3110-7.
Song X, Mtnitski A, Rockwood K (2010) Prevalence and 10-Year Outcomes of Frailty in Older Adults in Relation to Deficit Accumulation. J Am Geriatr Soc, 58 (4): 681-7.
Sternberg S, Wershof Schwartz A, Karunananthan S et al. (2011) The Identification of Frailty: A Systematic Literature Review. J Am Geriatr Soc, 59(11): 2129-38.
Sündermann S, Dademasch A, Rastan A et al. (2011) One-year follow-up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact CardioVasc Thorac Surg, 13(2): 119-23.
Waltson J, McBurnie M, Newman A et al. (2002) Frailty and Activation of the inflammation and coagulation systems with and without clinical co-morbidities: results from the Cardiovascular Health Study. Arch Intern Med, 162: 2333-41.
PubMed↗
Whitson H, Purser J, Cohen H (2007) Frailty thy name is … Phrailty? J Gerontol
A Biol Sci Med Sci, 62(7): 728–30.
PMID: 17634319
Woods N, LaCroix A, Gray S et al. (2005) Frailty: Emergence and consequences
in women aged 65 and older in the Women’s Health Initiative Observational Study.
J Am Geriatr Soc, 53(8): 1321–30.
PMID: 16078957
Xue QL (2011) The Frailty Syndrome: Definition and Natural History. Clin Geriatr Med, 27(1): 1-15.
PMID: 21093718