ICU Management & Practice, Volume 24 - Issue 4, 2024
Physical therapy is essential for improving outcomes and quality of life in solid organ transplant patients. This paper outlines the fundamental principles ("ABCs") of physical therapy, focusing on evidence-based practices and pre- and post-transplant care to guide healthcare professionals in optimising recovery.
Introduction
Physical therapy is essential in the postoperative care of patients who have undergone solid organ transplant (SOT), including those involving the kidney, liver, heart, or lungs. The primary objectives of physical therapy are to improve the quality of life and enhance clinical outcomes by emphasising physical rehabilitation, preventing post-surgery complications, and promoting cardiovascular-pulmonary readaptation (Sen et al. 2019). Personalised interventions, such as respiratory exercises and muscle strengthening, are used to strike a balance between rest and physical activity. This approach helps prevent muscle atrophy and other negative effects associated with prolonged immobility (Hoogeboom et al. 2014).
Physical therapy works in close coordination with the medical team to adapt the rehabilitation plan according to the patient’s evolving condition and their response to immunosuppressive therapy. This collaborative approach not only accelerates recovery but also increases patient autonomy and reduces both the length of hospital stays and readmission rates (Lemanu et al. 2013). As a result, physical therapy is crucial for ensuring the long-term success of organ transplants, facilitating more effective recovery, and extending the viability of the transplanted organ (Reese et al. 2014; Painter et al. 2001).
Prehabilitation for Solid Organ Transplant
Major surgeries, including solid organ transplants (SOTs), can lead to a reduction in functional capacity of up to 40% due to preoperative inactivity. This decreased functional capacity contributes to diminished physiological reserves and muscle atrophy, which impairs the body's ability to manage the stress associated with transplantation and achieve allostasis (Quint et al. 2023). Therefore, it is essential for candidates undergoing SOTs to be in optimal health to improve their resilience and reduce the risk of postoperative complications.
Prehabilitation aims to improve the overall physical condition of patients before surgery through a combination of exercise, dietary modifications, cognitive strategies, and psychosocial support. Most prehabilitation programmes, which typically last between 6 and 12 weeks, focus on aerobic exercise and functional strengthening (Lemanu et al. 2013). Programmes that are shorter in duration may not achieve the desired outcomes, underscoring the need to meticulously adjust factors such as duration, intensity, nutrition, and rest to enhance programme effectiveness (Takahashi et al. 2018).
In general, patients undergoing SOTs should be assessed for the possibility of early extubation (<3-8 hours), except in the case of lung transplants, which require more specific criteria. The decision to withdraw mechanical ventilation (MV) should take into account the transplanted organ, the patient’s stability, the progress of the surgical procedure, and the likelihood of success (Ragonete-dos-Anjos et al. 2022; Bilbao et al. 2003). Particularly for heart and lung transplants, extubation should not be rushed and must be closely monitored due to the complex interactions between the transplanted organ, the ventilator, and potentially extracorporeal support.
If early extubation is not possible, the critical care rehabilitation team will play a crucial role. Strategies such as early mobilisation, inspiratory muscle training, bronchial hygiene techniques, and respiratory care should be implemented (Hoogeboom et al. 2014). The multidisciplinary team's focus should be on optimising MV and maintaining or improving the patient's physical and respiratory condition to achieve a successful extubation (Ragonete-dos-Anjos et al. 2022).
Post-Transplant Management and Follow-Up
Although transplant patients can be managed similarly to other postoperative patients, understanding the specific complications and mortality risks associated with transplantation allows for a more targeted approach to care (Black et al. 2018). Key complications to monitor in this population include infections, cardiovascular issues, and pulmonary complications (Kinnunen et al. 2018; Piskin et al. 2022; Sen et al. 2019; Zelle et al. 2011). To streamline management, the following mnemonic —ABCs of transplant patient care— has been developed (Figure 1).
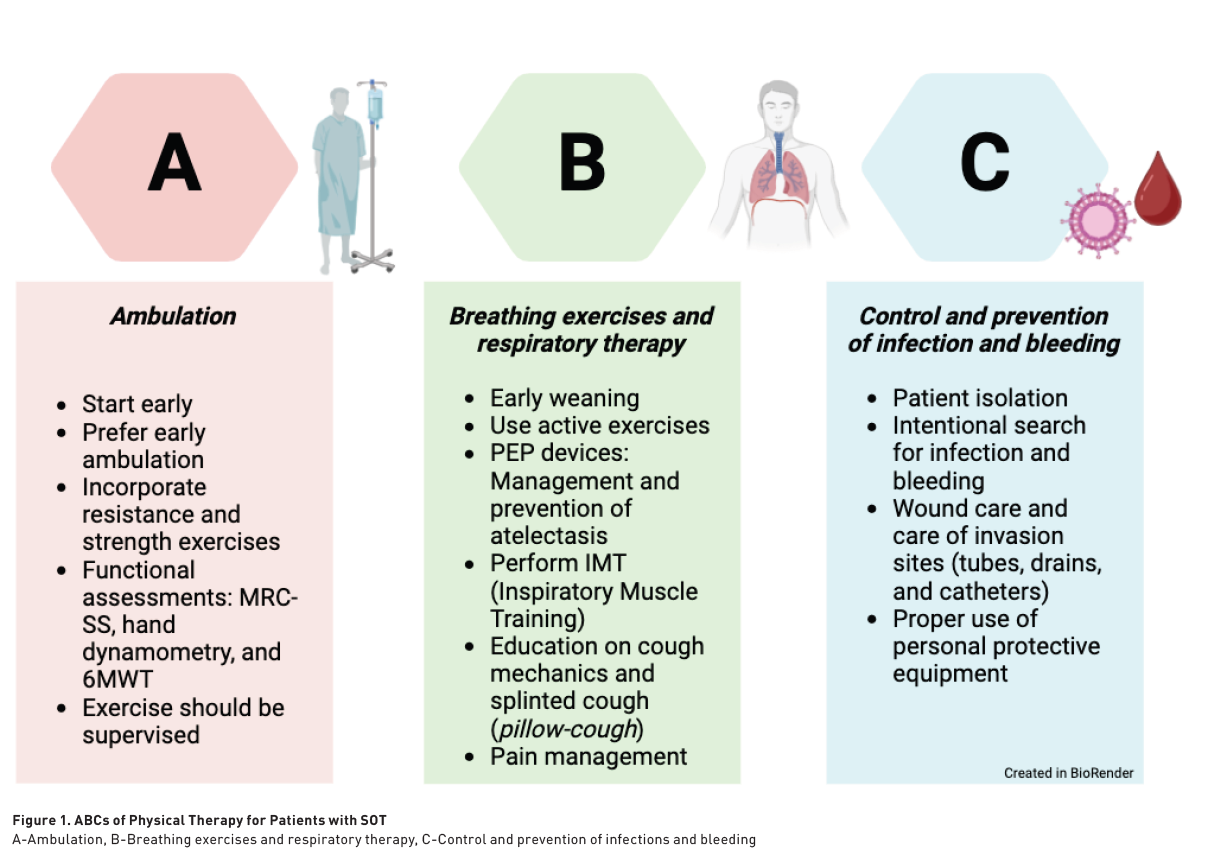
Ambulation
Physical conditioning plays a crucial role in the pulmonary rehabilitation of patients undergoing SOTs. The benefits include enhanced cardiopulmonary performance, improved survival prediction rates, and reduced hospital stays for liver transplants. Additionally, conditioning leads to increased VO2 max in patients undergoing cardiac, hepatic, and renal transplants (Bartels et al. 2011; Berben et al. 2019). Furthermore, this intervention has led to fewer in-hospital complications and improvements in quality of life and pain levels (Santa-Mina et al. 2015; Dunn et al. 2020).
Post-transplant physical rehabilitation programmes should be initiated early and supervised by physical therapists to maximise clinical benefits. Professional supervision ensures higher exercise intensity, optimised dosing and monitoring, leading to better clinical outcomes compared to self-directed supervision. There are several approaches for dosing exercise and advancing rehabilitation in patients following SOTs. Achieving the ability to walk and ambulate is a crucial functional milestone, as it enables more intensive training and the completion of the six-minute walk test. In addition, muscle strength training is essential for reducing frailty and improving functional capacity (Martins et al. 2020; Lands et al. 1999).
For patients undergoing SOTs, the most critical functional tests are the six-minute walk test and the sit-to-stand test (Quint et al. 2023; Ross et al. 2016; ATS Statement 2002; ATS/ACCP Statement 2002). These assessments are instrumental in estimating, monitoring, and enhancing functional capacity, as well as facilitating the safe dosing of exercise. Additionally, the Medical Research Council (MRC) scale and hand dynamometry are vital for evaluating and tracking muscle strength, and they are key components of the frailty assessment criteria for these patients.
Exercise interventions in clinical practice encompass a variety of approaches and methods aimed at improving the health and well-being of both hospitalised and outpatient patients. Common interventions include cycle-ergometry, functional exercises (such as active transfers to a chair), walking, and resistance training. The SEPAR guidelines underscore the importance of continuing respiratory exercises and promptly initiating seated activities and ambulation following the normalisation of any major surgery (López-Fernández et al. 2023).
Breathing Exercise and Respiratory Therapy
Post-transplant pulmonary care is comparable to that required after any major surgical procedure. Although the incentive spirometer (IS) was once widely utilised, recent evidence does not support its continued use (Larsen et al. 2022). While the device is not harmful and was a useful tool for surgeons before the adoption of mobilisation and respiratory physiotherapy protocols, its role has diminished. Currently, it is the physiotherapist's responsibility to implement protocols and train hospital staff in up-to-date pulmonary care practices for postoperative patients.
Additional respiratory interventions are available for the management of these patients. Positive expiratory pressure (PEP) devices and inspiratory muscle training (IMT) are commonly used. Oscillatory PEP devices facilitate the mobilisation of tracheobronchial secretions, whereas linear resistance PEP devices are employed to prevent and treat atelectasis (López-Fernández et al. 2023). IMT, which is intended to improve inspiratory muscle strength, is adjusted according to the patient's maximum inspiratory pressure (PiMax). The initial resistance of the device is set at 30-40% of the PiMax and can be progressively increased to 50-60% to optimise training outcomes in postoperative patients (Lemanu et al. 2013).
Finally, cough assistance techniques should start with thorough patient education on cough mechanics and secretion mobilisation. Commonly used methods, such as supported coughing or pillow-cough (where a pillow is placed on the surgical site to provide support and manual restriction during coughing) and effective post-coughing clearance, are generally sufficient for achieving proper expectoration. Induced coughing or chest compression techniques for accelerating expectoration are usually not recommended due to the pain at the surgical site; this pain should be managed with analgesia to facilitate effective cough mechanics (Larsen et al. 2022). The use of cough-assist devices is infrequent and is typically reserved for patients who have significant difficulty coughing or those experiencing pulmonary infections that result in excessive tracheobronchial secretions (López-Fernández et al. 2023).
Control and Prevention of Infection and Bleeding
Post-transplant isolation is essential for safeguarding immunosuppressed patients from infections (Black et al. 2018). During this period, the patient’s immune system, compromised by the immunosuppressive therapy required to prevent organ rejection, is highly susceptible to infections. Isolation minimises exposure to pathogens, facilitating the adaptation of the transplanted organ and allowing the immune system to recover gradually. The duration and intensity of isolation depend on the type of transplant and the patient’s overall health status. Effective isolation is crucial for ensuring the long-term success of the transplant and optimising the patient’s recovery.
Healthcare personnel must maintain heightened vigilance and actively identify potential sources of infection, which can manifest as symptoms such as fever, pain, wound discharge, foul odour, and leucocytosis (Kinnunen et al. 2018). Any suspected infection should be reported immediately to the medical team. Adherence to hand hygiene and the use of masks is mandatory during patient care. Likewise, educating patients and their families on preventive measures is crucial to avoid readmissions and ensure effective post-hospitalisation care.
Finally, similar to infection care, early monitoring and identification of postoperative bleeding will be a role that every physiotherapist working with this population should consider. Observing drains and probes, as well as closely monitoring patients' haemoglobin levels, are important data points to review (Faria et al. 2023). The presence of bleeding may be one of the criteria for considering the suspension of rehabilitation until the bleeding is stabilised or resolved.
Conclusion
In summary, physical therapy plays a pivotal role in the recovery of patients following solid organ transplants. It significantly enhances functional outcomes, mitigates complications, and improves overall quality of life. Implementing early exercise programmes and conducting meticulous monitoring are key strategies for optimising long-term results. Effective multidisciplinary collaboration and a patient-centred approach are critical to achieving successful outcomes.
“While we are all mortal, through organ donation, we become eternal”.
Dr Rivera Durón
Conflict of Interest
None.
References:
American Thoracic Society Statement (2002) Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 166:111e7.
American Thoracic Society/American College of Chest Physicians (2003) ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 167(2):211-77.
Argüero-Sánchez R, Sánchez-Ramírez O, Olivares-Durán EM (2020). Donación de órganos y trasplantes en México, ¿todo está resuelto? Gaceta médica de México. 156(3):181-183.
Bartels MN, Armstrong HF, Gerardo RE et al. (2011) Evaluation of pulmonary function and exercise performance by cardiopulmonary exercise testing before and after lung transplantation. Chest. 140(6):1604-1611.
Berben L, Engberg SJ, Rossmeissl A et al. (2019) Correlates and out- comes of low physical activity posttransplant: a systematic review and meta-analysis. Transplantation.103:679-688.
Bilbao I, Armadans L, Lazaro JL et al. (2003) Predictive factors for early mortality following liver transplantation. Clin Transplant. 17:401-411.
Black CK, Termanini KM, Aguirre O et al. (2018) Solid organ transplantation in the 21(st) century. Ann Transl med. 6(20):409.
De Smet S, Van Craenenbroeck AH (2021) Exercise training in patients after kidney transplantation. Clinical kidney journal. 14(Suppl 2):ii15–ii24.
Dunn MA, Rogal SS, Duarte-Rojo A, Lai JC (2020) Physical Function, Physical Activity, and Quality of Life After Liver Transplantation. Liver Transpl. 26(5):702-708.
Faria L, Fraga R, De-Souza D et al. (2022) Hemodynamic impact of early mobilization in critical patients receiving vasoactive drugs: A prospective cohort study. PLoS ONE. 17(12):e0279260.
Garcia AM, Veneroso CE, Soares DD et al. (2014) Effect of a physical exercise program on the functional capacity of liver transplant patients. Transplantation proceedings. 46(6):1807-1808.
Hoogeboom TJ, Dronkers JJ, Hulzebos EH, van Meeteren NL (2014) Merits of exercise therapy before and after major surgery. Current opinion in anaesthesiology. 27(2):161-166.
Kinnunen S, Karhapää P, Juutilainen A et al. (2018) Secular trends in infection-related mortality after kidney transplantation. Clin JAm Soc Nephrol. 13:755-62.
Lai JC, Sonnenday CJ, Tapper EB et al. (2019) Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 19:1896–1906.
Lands LC, Smountas AA, Mesiano G et al. (1999) Maximal exercise capacity and peripheral skeletal muscle function following lung transplantation. J Heart Lung Transplant. 18(2):113-120.
Larsen T, Chuang K, Patel S, Betancourt J (2022) Things We Do for No Reason™: Routine use of postoperative incentive spirometry to reduce postoperative pulmonary complications. Journal of hospital medicine, 17(12):1010–1013.
Lemanu DP, Singh PP, MacCormick AD et al. (2013) Effect of preoperative exercise on cardiorespiratory function and recovery after surgery: a systematic review. World J Surg. 37:711-720.
López-Fernández D, Fraile-Olivero CA (2023) Manual de procedimientos de fisioterapia respiratoria en cirugía torácica. Sociedad Española de Neumología y Cirugía de Tórax, Manual 41.
Martins CA, França A, Dias RSC et al. (2020) Prevalence of sarcopenia in kidney transplants and their association with determinant factors of muscle homeostasis. Rev Assoc Med Bras. 66(9):1235-40.
Maury G, Langer D, Verleden G et al. (2008) Skeletal muscle force and functional exercise tolerance before and after lung transplantation: a cohort study. Am J Transplant. 8(6):1275-1281.
Painter P, Krasnoff J, Paul SM et al. (2001) Physical activity and health-related quality of life in liver transplant recipients. Liver Transpl. 7:213e9.
Painter PL, Hector L, Ray K et al. (2002) A randomized trial of exercise training after renal transplantation. Transplantation. 74:42-8.
Piskin T, Simsek A, Murat-Dogan S et al. (2022) Mortality after kidney transplantation: 10-year outcomes. Cirugia y cirujanos. 90(2):172-179.
Prentis JM, Manas DM, Trenell MI et al. (2012) Submaximal cardiopulmonary exercise testing predicts 90-day survival after liver transplantation. Liver Transplantation. 18(2):152-159.
Quint EE, Zogaj D, Banning LBD et al. (2021) Frailty and kidney transplantation: a systematic review and meta-analysis. Transplant Direct. 7(6):e701.
Quint EE, Ferreira M, van Munster BC et al. (2023) Prehabilitation in Adult Solid Organ Transplant Candidates. Curr Transplant Rep. 10(2):70-82.
Ragonete-dos-Anjos AP, Santana IF, Heidemann A et al. (2022) Factores de riesgo de reintubación relacionados con insuficiencia no relacionada con las vias respiratorias despues de un trasplante de hígado en la unidad de cuidados intensivos: estudio observacional. BJT. 25(01):e0222.
Reese PP, Bloom RD, Shults J et al. (2014) Functional status and survival after kidney transplantation. Transplantation. 97:189-195.
Reyes-Acevedo R, Obrador GT, Alberú-Gómez J et al. (2019) Current state and challenges for organ donation and transplantation in Mexico. Transplantation. 103:648-650.
Rongies W, Stepniewska S, Lewandowska M et al. (2011) Physical activity long-term after liver transplantation yields better quality of life. Ann Transplant. 16(3):126e31.
Ross R, Blair SN, Arena R et al. (2016) Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 134:e653-e699.
Santa Mina D, Scheede-bergdahl C, Gillis C, Carli F (2015) Optimization of surgical outcomes with prehabilitation. Appl Physiol Nutr Metab. 40(9):966-9.
Sen A, Callisen H, Libricz S et al. (2019) Complications of solid organ transplantation: cardiovascular, neurologic, renal, and gastrointestinal. Crit Care Clin. 35:169-186.
Takahashi A, Hu SL, Bostom A (2018) Physical activity in kidney transplant recipients: a review. Am J Kidney Dis. 72:433-443.
Thuluvath PJ, Yoo HY, Thompson RE (2003) A model to predict survival at one month, one year, and five years after liver transplantation based on pretransplant clinical characteristics. Liver Transpl. 9:527-532.
Zelle DM, Corpeleijn E, Stolk RP et al. (2011) Low physical activity and risk of cardiovascular and all-cause mortality in renal transplant recipients. Clin J Am Soc Nephrol. 6:898-905.












