ICU Management & Practice, Volume 16 - Issue 2, 2016
Guiding Vascular Access Selection for Intensive Care - a Summary of Michigan Appropriateness Guide for Intravenous Catheters (MAGIC)
Determining appropriateness for vascular access devices
limits the risk of complications in critically ill patients. Michigan Appropriateness
Guide to Intravenous Catheters (MAGIC) establishes evidence-based indications
as summarised in this paper.
Safe and reliable venous access is the foundation for medication
administration in critical and intensive care unit (ICU) patients. Several
important issues surround vascular access in the ICU setting, including the
need for multiple multi-lumen devices for delivery of concomitant drugs and the
frequent sampling of blood from catheters. Risk factors associated with
catheter-related complications in ICU patients are coma/immobility and the number
of catheters present (Villamarín-Bello et al. 2016). The risk of complications
associated with central venous catheters is higher in ICUs compared to other
departments, with 35% greater prevalence in one prospective study evaluating
peripherally inserted central catheters (Leroyer et al. 2013). Balancing the needs
of clinically unstable patients with risks associated with numerous vascular
devices requires a process for device selection, aseptic insertion, management
and removal of devices when no longer necessary.
Central venous access devices commonly used in ICUs pose
significant infectious and thrombotic risk to patients (Maki et al. 2006). Potential
risk factors identified as contributing to the development of infectious and
thrombotic complications are the patient’s underlying disease, type of
catheter, immobility, sedation and duration of catheter use (Richet et al. 1990).
The concern for thrombosis includes lower extremities for immobile patients,
but also heightened concern for upper extremity thrombosis from central venous
access devices (CVAD) (Kearon et al. 2012; Clemence and Maneval 2014). Central
devices inserted in the arm, such as peripherally inserted central catheters (PICCs),
have a higher risk of thrombosis, with incidence in the literature ranging from
2-75% (Chopra et al. 2013a; Clemence and Maneval 2014; Fallouh et al. 2015).
Increasing use of PICCs in intensive care has similarly led to greater levels
of thrombosis in this patient population (Chopra et al. 2013a). The association
between thrombosis, infections and central catheters highlights why use of
devices such as PICCs should be considered only when indicated (Evans et al.
2010; Chopra et al. 2012a; Chopra et al. 2013a; Chopra et al. 2013b; Malinoski
et al. 2013; Moureau 2013a; Marschall et al. 2014).
Guidance for selection with evidence-based indications for
PICCs or other chest-inserted central catheters (CICC) has been lacking despite
recommendations for hospitals to establish tighter criteria. The Society of
Healthcare Epidemiology of America (SHEA) recommends providing clinicians with
easy access to an evidence-based list of indications for CVC, prior to
placement, to minimise unnecessary central catheters and limit risk of central
line-associated bloodstream infections (CLABSI) (Marschall et al. 2014). In an
effort to address the issues and potentially reduce vascular access device risk
to patients, a multidisciplinary panel of national and international experts
was convened to examine criteria for appropriate placement of peripherally
inserted central catheters (PICCs) in comparison with other peripheral and
central venous devices (Chopra et al. 2015). The Michigan Appropriateness
Guide for Intravenous Catheters (MAGIC): Results from a
Multispecialty Panel Using the RAND/UCLA Appropriateness Methodreflects the in-depth evaluation of vascular
access devices to provide the evidence needed to guide selection (Chopra, Flanders
et al. 2015).
Methods
MAGIC was formulated using the RAND Corporation/University
of California Los Angeles (RAND/UCLA) Appropriateness Method (Fitch et al.
2001). Following systematic reviews of the literature and compilation of
available evidence, clinical scenarios were created to rate the appropriateness
of insertion, maintenance and care of PICCs in comparison with other peripheral
and central venous access devices. Using a conceptual framework of categories such
as duration of use, type of infusate, patient, device and provider factors,
scenarios were developed for ratings. In accordance with the RAND/UCLA method,
the purpose of the panel was not to reach consensus, but rather evaluate why
disagreement occurred in order to minimise misunderstandings when rating each scenario. A multi-specialty group of experts was
selected to review the literature and rate the appropriateness of each of the
scenarios for each of the devices including peripherally inserted central
catheters (PICCs), ultrasonography-guided peripheral intravenous catheters, midline
catheters, and peripheral intravenous catheters, non-tunnelled CVCs, tunnelled
CVCs and ports.
Results of MAGIC
A summary of appropriate and inappropriate vascular access
applications follows and is condensed in Table 1 Vascular Access Dashboard.
For more detailed information on the results of MAGIC refer to the complete
publication (Chopra et al. 2015).
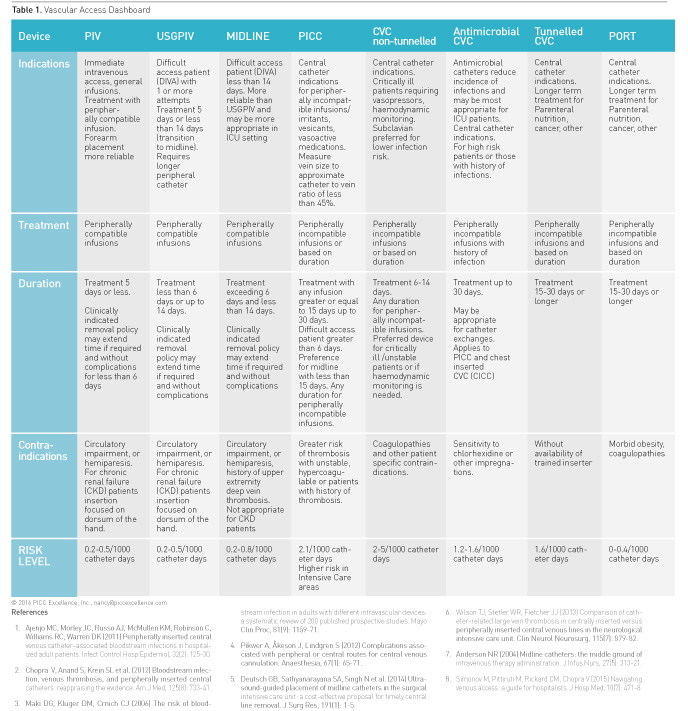
Peripherally Inserted Central Catheters (PICCs)
Peripherally inserted central catheters (PICCs) are
currently used in all care settings with a reported volume of 2.9 million per
year used in the USA market alone (iData Research 2014). Specific indications
for PICCs in intensive care areas include administration of vasopressors, delivery
of peripherally incompatible infusions, parenteral nutrition, frequent blood
sampling of three times a day or more, need for invasive haemodynamic
monitoring, or patients who may require infusions greater than 15 days (Table
1 Vascular Access Dashboard). Importantly several studies (including a
recent randomised trial and a meta-analysis of 64 studies) suggest that the
risk of upperextremity thrombosis is higher for PICCs in critically ill
patients (Chopra et al. 2013a). For this reason, non-tunnelled CVCs are rated
as appropriate for use in ICU settings over PICCs when such use is proposed to
last <14 days. In patients with chronic kidney disease (CKD) (glomerular
filtration rate of less than 45 mL/min, creatinine level greater than 3.0,
those on dialysis or with stage 3b CKD or greater) peripheral access with PICCs
is considered inappropriate and should be preceded by nephrology consultation
(Hoggard et al. 2008; Drew and Weiner 2016). In patients with difficult access
and no central infusion indications, MAGIC recommendations list a preference for
ultrasound-guided peripheral catheters or midline devices rather than PICCs.
Short Peripheral, Ultrasound-Guided Peripheral and Midline Catheters
Indications for short peripheral catheters include immediate
intravenous access for peripherally compatible infusions with treatment duration
of 5 days or less. Short peripheral catheters are available in 1-6cm lengths with
the longer 4-6cm catheters used with ultrasound-guided deeper catheter
insertions. Specialists are often called upon when peripheral catheters fail or
when multiple peripheral cannulation attempts are required (Helm et al. 2015).
Ultrasound-guided peripheral catheters (USGPIV) are indicated for patients with
difficult intravenous access (DIVA), defined as patients having one or more
failed cannulation attempts. USGPIV or midlines are beneficial when central
access devices are no longer necessary or indicated. Reports demonstrate 92-99%
success with USGPIV cannulation when education, supervised insertions and competency
assessment are established for inserters (Chinnock et al. 2007; Mills et al. 2007;
Bauman et al. 2009; Gregg et al. 2010; White et al. 2010; Witting et al. 2010;
Moureau 2013; Deutsch et al. 2014). In one study of 148 USGPIV insertions, 40
CVADs were discontinued and 34 CVADs avoided with placement of peripheral
catheters using ultrasound guidance (Gregg et al. 2010).
While ultrasound can be used to place any intravenous
catheter, we use the term USGPIVs to refer to the ultrasound needle-guided
placement of catheters of greater length (4-6cm), owing to the greater depth
needed for access (Keyes et al. 1999). USGPIV are appropriate for difficult
access patients requiring treatment for 6 or fewer days or up to 14 days with
peripherally compatible infusions. Midline catheters provide even greater
catheter length for longer dwell. Midline catheters range from 8-20cm in length
with the terminal tip in the basilic, brachial or cephalic veins. Notably
midlines should not extend into the axillary vein or enter the chest (Gorski et
al. 2016). Indications for midline catheters mirror USGPIV for indications of
treatment up to 14 days. Additionally midlines may be a more reliable peripheral
catheter for intensive care patients, owing to their longer dwell time and more
stable upper arm placement (Anderson 2004; Mills et al. 2007; Garcia 2009;
Alexandrou et al. 2011; Morrison 2012; Warrington et al. 2012; Baliad and
Peterson 2013; Dawson and Moureau 2013). A policy ensuring that peripheral
catheters are removed when clinically indicated rather than on a routine basis
is also recommended by MAGIC. (Rickard et al. 2012; Webster et al. 2013;
Tuffaha et al. 2014).
Chest Inserted Central Catheters (CICC)
MAGIC examined the appropriateness of nontunnelled chest
inserted central catheters, tunnelled catheters, as well as subcutaneously implanted
ports in comparison with PICCs. Based on treatment, the peripheral
compatibility of the infusate, proposed duration of infusion and other factors
dictating the need for central administration, the use of nontunnelled acute
care catheters for 6-14 days was considered appropriate. Non-tunnelled catheters
are preferred over PICCs when risk factors for thrombosis are present or when there
is a history of deep vein thrombosis (Chakravarthy et al. 2005; Evans et al.
2010; Chopra et al. 2013a). Preference was given for non-tunnelled CVADs for patients who were haemodynamically
unstable, actively receiving vasopressors or requiring urgent central venous access
(Chopra et al. 2015). Tunnelled catheters were indicated when at least 3 months
of treatment were needed. Ports were considered appropriate for treatment that
required intravenous access for 6 months or more and neutral for treatment of
3-6 months.
Conclusion
Maintaining vascular access is a top priority in the
intensive care patient population. The selection of vascular access devices for
critically ill patients requires the clinician to consider many factors that
impact patient risk and safety. With prolonged immobility and critical illness,
the risk of thrombosis and infection must be factored into the equation when
selecting a device. Selection criteria established within the MAGIC guide can
help determine which device is associated with least risk and meets treatment
needs of the patient (Anderson and Spencer 2003; Maki et al. 2006; Crowley et al.
2008; Chopra et al. 2012b; Clemence and Maneval 2014; Chopra et al. 2015).
MAGIC provides guidance and measurement criteria through which to assess the
appropriateness of PICCs and other vascular access devices for the intensive
care patient (Chopra et al. 2015; Woller et al. 2015). Application of MAGIC by clinicians
and providers within intensive care areas may assist hospitals in establishing
reliable access, improving outcomes, achieving infection prevention goals and
reducing burden of thrombosis.
Conflict of Interest
Nancy L. Moureau is the chief executive officer of PICC
Excellence, Inc., a speaker and educational consultant with 3M, Access
Scientific, Angiodynamics, Arrow/Teleflex, BD Carefusion, Chiesi, Cook,
Entrotech, Excelsior, Fresenius Kabi, and Nexus; a research doctoral candidate
with the Alliance for Vascular Access Teaching and Research at Griffith
University, and clinician at Greenville Memorial University Medical Center.
Vineet Chopra declares that he has no conflict of interest.
Abbreviations
CICC chest inserted central catheter
CKD chronic kidney disease
CLABSI central line-associated bloodstream infections
CVAD central venous access devices
CVC central venous catheter
DIVA difficult intravenous access
ICU intensive care unit
PICC peripherally inserted central catheters
MAGIC Michigan Appropriateness Guide for Intravenous Catheters
USGPIV ultrasound-guided peripheral catheters
References:
Alexandrou E, Ramjan LM , Spencer T et al. (2011) The use of midline catheters in the adult acute care setting – clinical implications and recommendations for practice. The Journal of the Association for Vascular Access, 16(1): 35-8, 40-1. Article ↗
Anderson FA Jr, Spencer FA (2003) Risk factors for venous thromboembolism. Circulation, 107(23 Suppl 1): 16-9. PubMed ↗
Anderson NR (2004) Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs 27(5): 313-21.PubMed ↗
Baliad P, S Peterson (2013) Midline catheter reduces infiltrations for coronary artery bypass graft patients. Poster presented at Infusion Nurses Society 2013 Annual Convention & Industrial Exhibition, 18-23 May 2013, Charlotte, NC, USA.
[Accessed: 19 April 2016] Available from accessscientific.com/media/v.-Baliard-Poster-Midline-Catheter-Reduces-Infiltration-for-Coronary-Artery-Bypass-Graft-Patients.pdf Article ↗
Bauman M, Braude D, Crandall C (2009) Ultrasound-guidance vs. standard technique in difficult vascular access patients by ED technicians. Am J Emerg Med, 27(2): 135-40.PubMed ↗
Chakravarthy S, Rettmann J, Markewitz B et al. (2005) Peripherally inserted central catheter (PICC) associated upper extremity deep venous thrombosis (UEDVT) in critical care setting. Chest, 128 (4_MeetingAbstracts): 193S–4S Article ↗
Chinnock B, Thornton S, Hendey GW (2007) Predictors of success in nurse-performed ultrasound-guided cannulation. J Emerg Med, 33(4): 401-5.PubMed ↗
Chopra V, Flanders SA, Saint S (2012a) The problem with peripherally inserted central catheters. JAMA, 308(15): 1527-8.
PubMed ↗
Chopra V, Anand S, Krein SL et al. (2012b) Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med, 125(8): 733-41.PubMed ↗
Chopra V, Anand S, Hickner A et al. (2013a) Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet, 382(9889): 311-25.PubMed ↗
Chopra V, O'Horo JC, Rogers MA et al. (2013b) The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol, 34(9): 908-18.PubMed ↗
Chopra V, Flanders SA, Saint S et al. (2015) The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med, 163(6 Suppl): S1-40.PubMed ↗
Clemence BJ, Maneval RE (2014) Risk factors associated with catheter-related upper extremity deep vein thrombosis in patients with peripherally inserted central venous catheters: literature review: part 1. J Infus Nurs, 37(3): 187-96.PubMed ↗
Crowley A, Peterson G, Benjamin D et al. (2008) Venous thrombosis in patients with short-and long-term central venous catheter-associated Staphylococcus aureus bacteremia. Crit Care Med, 36(2): 385-90.PubMed ↗
Dawson R, Moureau N (2013) Midline catheters: an essential tool in CLABSI reduction. Infection Control Today. [Online] [Accessed: 19 April 2016]. Available from infectioncontroltoday.com/articles/2013/03/midline-catheters-an-essential-tool-in-clabsi-reduction.aspx Article ↗
Deutsch GB, Sathyanarayana SA, Singh N et al. (2014) Ultrasound-guided placement of midline catheters in the surgical intensive care unit: a cost-effective proposal for timely central line removal. J Surg Res, 191(1): 1-5.PubMed ↗
Drew DA, Weiner DE (2016). Peripherally Inserted central catheters (PICCs) in CKD: PICC’ing the best access for kidney disease patients. Am J Kidney Dis, pii: S0272-6386(16)00082-2. doi: 10.1053/j.ajkd.2016.01.013. [Epub ahead of print] PubMed ↗
Evans S, Sharp J, Linford L et al. (2010) Risk of symptomatic DVT associated with peripherally inserted central catheters. Chest, 138(4): 803-10.PubMed ↗
Fallouh N, McGuirk HM, Flanders SA et al. (2015) Peripherally inserted central catheter-associated deep vein thrombosis: a narrative review. Am J Med, 128(7): 722-38.PubMed ↗
Fitch K, Bernstein SJ, Aguilar MD et al. (2001) The RAND/UCLA appropriateness method user's manual. [Accessed: 19 April 2016]. Available from rand.org/content/dam/rand/pubs/monograph_reports/2011/MR1269.pdf Article ↗
Garcia R (2009) The Midline intravenous catheter: meeting the challanges of patient safety and cost control. ICT: 38-46. [Online] [Accessed: 19 April 2016]. Available from Article ↗
Gorski L, Hadaway L, Hagle M et al. (2016) Infusion therapy: standards of practice (Supplement 1). J Infus Nurs, 39(1S): S1-S159.
Gregg SC, Murthi SB, Sisley AC et al. (2010) Ultrasound-guided peripheral intravenous access in the intensive care unit. J Crit Care, 25(3): 514-9.PubMed ↗
Helm RE, Klausner JD, Klemperer JD et al. (2015) Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs, 38(3): 189-203.PubMed ↗
Hoggard J, Saad T, Schon D et al. (2008) Guideline for venous access in patients with chronic kidney disease. A Position Statement from the American Society of Diagnostic and Interventional Nephrology Clinical Practice Committee and the Association for Vascular Access. Seminars in Dialysis, 21(2): 186-91.PubMed ↗
iData Research (2014) US vascular access devices market - 2014. Accessed: 20 April 2016]. Available from idataresearch.com/u-s-vascular-access-devices-market-2014 Article ↗
Kearon C, Akl EA, Comerota AJ et al. (2012) Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest, 141(2 Suppl): e419S-94S.PubMed ↗
Keyes LE, Frazee BW, Snoey ER et al. (1999) Ultrasound-guided brachial and basilic vein cannulation in emergency department patients with difficult intravenous access. Ann Emerg Med, 34(6): 711-4.PubMed ↗
Leroyer C, Lasheras A, Marie V et al. (2013) Prospective follow-up of complications related to peripherally inserted central catheters. Med Mal Infect, 43(8): 350-5.PubMed ↗
Maki DG, Kluger DM, Crnich CJ (2006) The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc, 81(9): 1159-71.PubMed ↗
Malinoski D, Ewing T, Bhakta A et al. (2013) Which central venous catheters have the highest rate of catheter-associated deep venous thrombosis: a prospective analysis of 2,128 catheter days in the surgical intensive care unit. J Trauma Acute Care Surg, 74(2): 454-62. PubMed ↗
Marschall J, Mermel L, Fakih M et al. (2014) Strategies to Prevent Central Line–Associated Bloodstream Infections in Acute Care Hospitals: 2014 Update. Infect Control Hosp Epidemiol, 35(7): 753-71.PMID: 24915204 PubMed ↗
Mills CN, Liebmann O, Stone MB et al. (2007) Ultrasonographically guided insertion of a 15-cm catheter into the deep brachial or basilic vein in patients with difficult intravenous access. Ann Emerg Med, 50(1): 68-72.PubMed ↗
Morrison T (2012) Qualitative analysis of central and midline care in the medical/surgical setting. Clin Nurse Spec, 26(6): 322-8.PubMed ↗
Moureau N (2013a) Catheter-related infection and thrombosis: a proven relationship - a review of innovative PICC technology to reduce catheter-related infection and thrombosis. [Online] [Accessed: 20 April 2016]. Available from chlorhexidinefacts.com/docs/Relationship_of_Catheter_Related_Infection_Thrombosis_WP_2012-1261.pdf Article ↗
Moureau N (2013b) US PIV Midline insertion competency assessment. PICC Excellence, Inc. [Accessed: April 2 2016]. Available from piccexcellence.com Article ↗
Richet H, Hubert B, Nitemberg G et al. (1990) Prospective multicenter study of vascular-catheter-related complications and risk factors for positive central-catheter cultures in intensive care unit patients. J Clin Microbiol, 28(11): 2520-5.PubMed ↗
Rickard CM, Webster J, Wallis MC et al. (2012) Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet, 380(9847): 1066-74.PubMed ↗
Tuffaha HW, Rickard CM, Webster J et al. (2014) Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Health Policy, 12(1): 51-8.PubMed ↗
Villamarín-Bello B, Piñeiro-Lamas M, Barros-Dios JM et al. (2016) Bacteremia nosocomial asociada a catéter vascular central en unidades de cuidados intensivos en 2 hospitales en Galicia (España). Infectio, 20(2): 62-9. Article ↗
Warrington WG, Aragon A, Penoyer T et al. (2012) Outcomes of using a modified Seldinger technique for long term intravenous therapy in hospitalized patients with difficult venous access. J Assoc Vasc Access, 17(1): 24-30.Article ↗
Webster J, Osborne S, Rickard CM et al. (2013) Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev (4): CD007798.PubMed ↗
White A, Lopez F, Stone P (2010) Developing and sustaining an ultrasound-guided peripheral intravenous access program for emergency nurses. AENJ, 32(2): 173-88. Article ↗
Witting MD, Schenkel SM, Lawner BJ (2010) Effects of vein width and depth on ultrasound-guided peripheral intravenous success rates. J Emerg Med, 39(1): 70-5.PubMed ↗
Woller SC, Stevens SM, Evans RS (2015) The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC) initiative: a summary and review of peripherally inserted central catheter and venous catheter appropriate use. J Hosp Med, 11(4):306-10. PubMed ↗








