HealthManagement, Volume 15 - Issue 3, 2015
Political Background
Safety and quality have been highlighted by the European Commission’s Directorate-General for Health and Food Safety (European Commission Expert Panel on Effective Ways of Investing in Health Expert Panel 2014). The Expert Panel on Effective Ways of Investing in Health was tasked with considering the core dimensions of quality of healthcare, including patient safety.
The Expert Panel listed the dimensions of safety and related goals:
- Development of safety systems (including authorities, bodies, culture of patient safety, standards/ guidelines) and strategies (policies, programmes); • Development of patient safety information and learning systems;
- Education and training of healthcare workers, management and administrative staff;
- Encouragement of multidisciplinary patient safety on-the-job education and training;
- Empowering and informing citizens and patients, including patient involvement in safety policies.
The Panel noted that the most frequently used dimensions of quality of care include safety. However, these dimensions are not mutually exclusive and cannot be considered comprehensive (see Table 1).
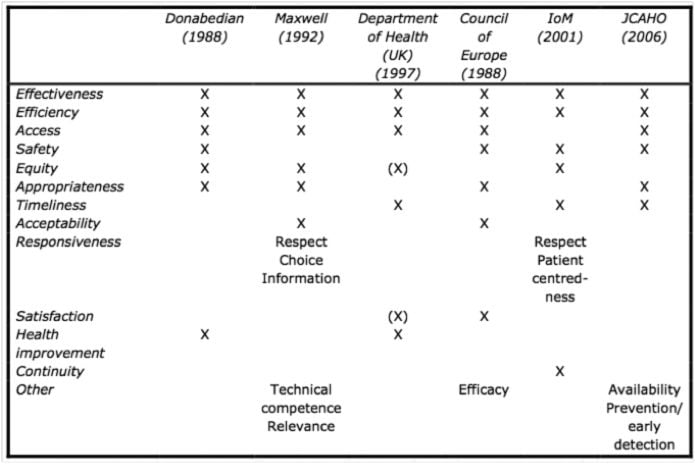
Table 1. Dimensions of Quality of Care
Source: Expert Panel on Effective Ways of Investing in Health (2014)
The European Society of Radiology’s Call for a European Action Plan for Medical Imaging to Improve Quality of Care and Patient Safety (ESR 2014) was launched i n November 2 014 to target policy-makers to strengthen harmonisation efforts in regard to quality and safety, education and t raining, as well as research and technology, in order to significantly improve European healthcare systems and ensure better quality and safety for European patients.
To progress harmonisation of safety in imaging across Europe, the ESR calls on the EU institutions to:
• Support the establishment of European quality and safety indicators for imaging;
• Support an audit of imaging equipment, doses, image quality and procedures of the medical imaging chain in Europe, and to develop plans to modernise equipment;
• Support efforts to improve communication with patients;
• Improve inter-institutional cooperation for more coherent action in the area of health;
• Support the EuroSafe Imaging campaign (eurosafeimaging.org) to raise awareness of the importance of radiation protection.
The Concept
Quality healthcare by definition means safe healthcare, and safety should be managed as an integral part of quality assurance. Safety, as defined by the National Patient Safety Foundation, is “the degree to which health care processes avoid, prevent, and ameliorate adverse outcomes or injuries that stem from the processes of health care itself” (National Patient Safety Foundation 2000). The Institute of Medicine defines it as freedom from accidental injury due to medical care or medical errors (Institute of Medicine 1999).
The EuroSafe Imaging initiative (eurosafeimaging. org) was set up in 2014 to promote quality and safety in medical imaging. The twin roles of quality and safety are summed up in Figure 1, showing that a process-oriented and patient-centred approach is integral to medical imaging quality and safety. The International Atomic Energy Agency (IAEA) issued its draft safety guide Radiation Protection Safety in Medical Uses of Ionizing Radiation in November 2014 (International Atomic Energy Agency 2014) for comments by member states due by the end of April 2015.
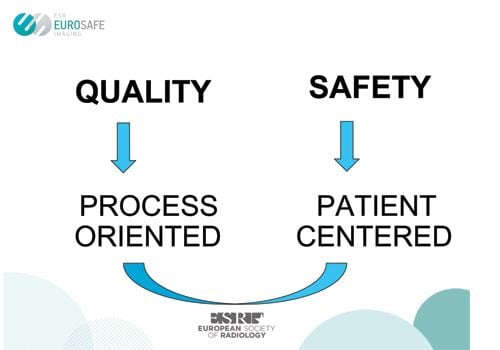
Figure 1. Relationship between Quality and Safety
Patient Information on Radiation Safety
To promote patient understanding of radiation risk, health professionals need to establish confidence with the patient, emphasise that potential risks are an estimation and not actual, use the concept of benefit instead of risk and explain the quality of the practice and the equipment.
Together with the ESR Patient Advisory Group (ESR-PAG), EuroSafe Imaging will be working on patient information on radiation risks to add to its website, and will provide benchmarking tools through dose surveys.
The American College of Radiology (ACR)’s and the Radiological Society of North America (RSNA)’s public information website radiologyinfo.org includes a section on patient safety, with information on radiology benefits and risks, radiation dose in x-ray and CT exams, and a printable medical imaging record card that patients can use to record their medical imaging history. In addition, the ACR has published a Position Statement on Quality Control and Improvement, Safety, Infection Control, and Patient Education (American College of Radiology 1998).
The University of California, San Francisco’s (UCSF) radiology department is an example of a well-developed radiation safety programme (radiology.ucsf. edu/patient-care/patient-safety) that includes an experienced faculty member who devotes much of their time to patient safety. The department’s website includes guidelines for use of CT and MRI during pregnancy and lactation as well as MRI and contrast guidelines.
They list ten ways to ensure imaging safety:
- Choosing the most appropriate imaging study;
- Tailored techniques;
- Careful quality control;
- Latest CT technology;
- Special attention for paediatric patients;
- New low-dose CT protocols;
- Shielding;
- Beam collimation policy;
- Appropriate training;
- Radiation oversight committee.
Clinical Audit
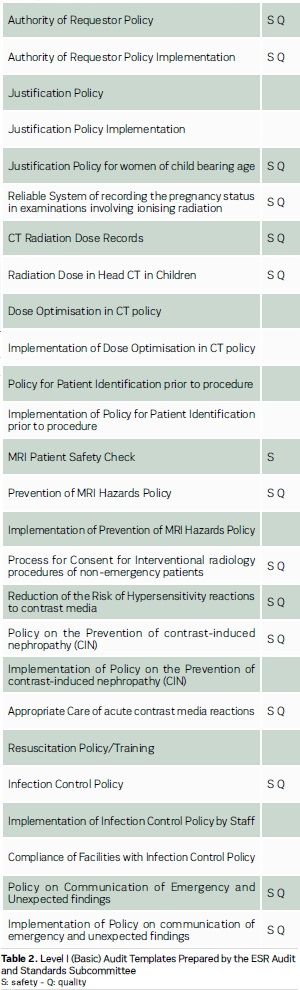
Under Directive 97/43 Euratom and its successor Directive 2013/59 Euratom, applicable from 2018 (Council Directive 2013), clinical audit on radiation safety is mandatory. The European Society of Radiology’s (ESR) Audit and Standards Subcommittee has therefore launched Level I (basic) audit templates in 2015, which address essential patient safety standards, and is preparing the Level II templates for release in 2016 (see Table 2).
Safety: Registries, Reporting
In Pennsylvania t here is a good example of safety reporting in a 2009 study that showed the following types of errors: (see Table 3) Errors do happen in the radiology department, with failure to correctly identify patients leading to recognised wrong events, the potential for treating the wrong patient, doing the wrong procedure on the wrong side or the wrong site.
The main errors are:
- Wrong examination;
- Wrong patient;
- Wrong side;
- Wrong site;
- Wrong contrast agent;
- MR safety;
- Wrong protocol;
- Pregnancy (technician/radiologist not aware that patient is pregnant).
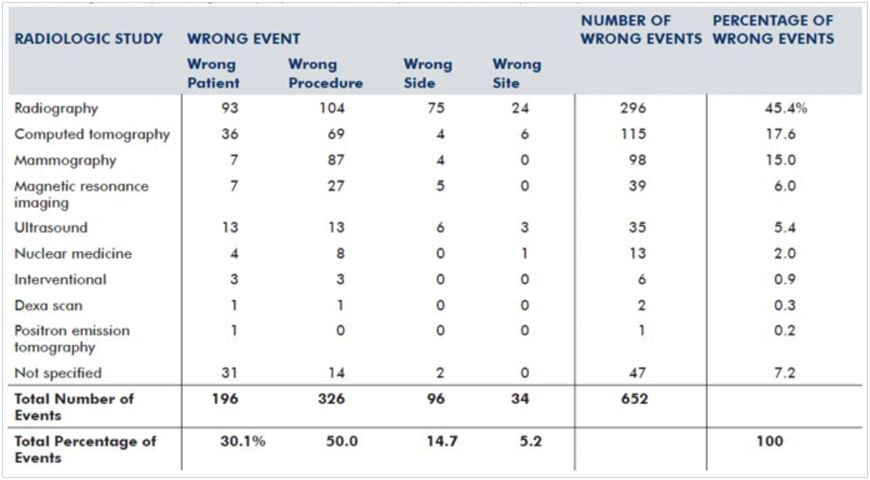
Table 3. Wrong events by radiologic study reported to the Pennsylvania Patient Safety Authority, 2009
Source: Pennsylvania Patient Safety Authority 2011
Such errors are caused by incorrect order or requisition entry, failure to confirm patient identity, failure to follow site and procedure verification or procedure qualification processes. Such errors can be prevented with clear procedures on MRI safety, identifying pregnancy and contrast agent procedures for iodinated agents and gadolinium chelates use.
Brook et al. (2010) found that poor communication, whether it was verbal communication or IT-related, caused many errors. Others have highlighted communication as the root of errors, for example:
- “Poor communication is at t he heart of many medical errors.” (Woolf et al. 2004).
- Communication failures t hat contribute to discontinuity of care stem from a variety of causes, ranging from a lack of interpersonal communication s kills to barriers in the work environment to suboptimal use of computer networking tools.” (Scott 2007).
Radiology departments should establish an events registry. One model is perhaps the U.S. Agency for Healthcare Research and Quality’s Patient Safety Indicators (PSIs) (n.d.) that provide information on potential in-hospital complications and adverse events following surgeries, procedures and childbirth. Another example is the Radiology Events Register, an Australian initiative (Mandel 2015). In Europe, imaging and quality safety indicators are being developed by the ESR with the goal of allowing standardised reporting and to aid safety improvement.
Clinically Justified Examinations
Doing clinically justified examinations is certainly a major pillar of safety. For example, the number of examinations per 1000 of population clearly shows a high discrepancy between countries (see Figure 2), indicating in some situations either overuse or underuse, both being unsafe practices. In this context, monitoring of clinical indications should be helpful for finding a good compromise. The launch of ESR iGuide, a clinical decision support system for European imaging referral guidelines, is intended to support the justification principle by providing electronic decision support to referring doctors ordering imaging tests.
The recently published study by Ip et al. (2015) found wide variations also in the United States, and identified potential targets for future imaging quality improvement initiatives, including head CT and lumbar spine MR imaging.
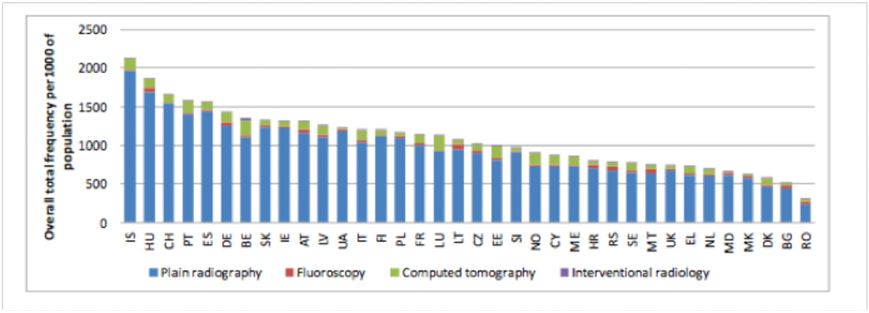
Figure 2. Example of Heterogeneity of Practice. Source: European Commission Directorate General for Energy (2014)
Furthermore, it is well known that radiation dose differs between technicians, radiologists, within departments and across countries even for a very simple examination like chest x-ray (see Figure 3).
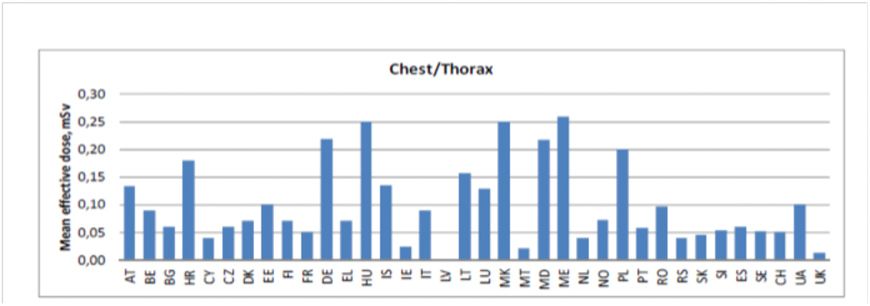
Figure 3. Variation of the Effective Dose for Chest X-Ray across Europe.Source:
European Commission Directorate General for Energy (2014)
The
establishment of standardised protocols and dose monitoring appears to be
essential in this context.
Patients’ and Professionals’ Awareness
Surprisingly, there is still little awareness of radiation risk from imaging procedures among healthcare professionals. Ramanathan and Ryan (2015) surveyed 92 residents, fellows, technologists and radiologists in a hospital group, and found that knowledge of radiation dose and risk is poor among all radiology workers. They found that for effective dose and for cancer risk in particular, the stated opinions of people working in imaging departments do not correctly reflect actual effective doses and cancer risk. (see Figure 4)
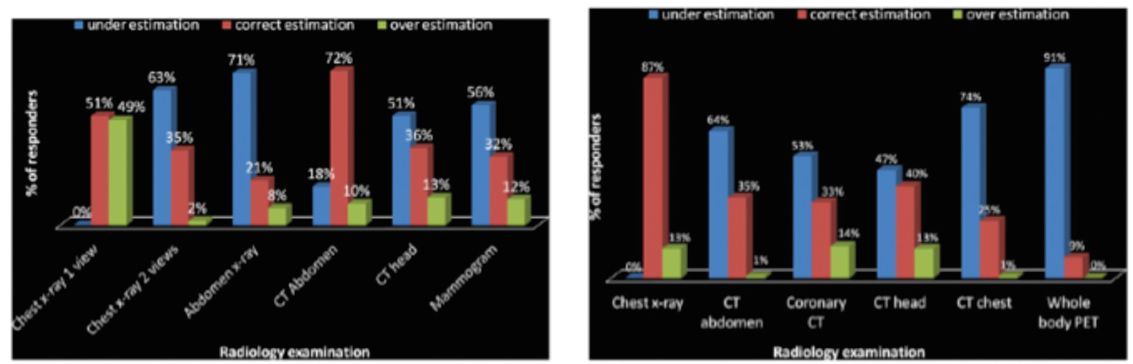
Figure 4. Radiation Risk Awareness
Percentage of participants who underestimated and overestimated effective dosage equivalents of different radiology examinations (shown left), and the cancer risk form different radiology examinations (shown right)
Source: Ramanathan and Ryan (2015)
Although this is a Canadian study, we cannot assume that awareness amongst health professionals is any better in Europe. There is a lot to be done in education, which is why EuroSafe Imaging provides e-learning materials and radiation protection sessions for health professionals. Action 8 of EuroSafe Imaging’s 12-point action plan is to develop a data collection project called “Is your Imaging EuroSafe?” and an educational project on guidelines entitled “Are you imaging appropriately?”.
The aims are to build a European repository based on dose exposures for specific clinical indications that would be most helpful for self-benchmarking and for future establishment of diagnostic reference levels (DRLs), to provide insights into how the age of the equipment affects dose exposure, and also to create a tool for communication with patients.
Data is being collected on the following CT procedures:
- CT head: acute stroke
- CT chest: pulmonary embolus
- CT head: acute head trauma
- CT chest: rule out pulmonary metastases of extrathoracic cancer
- CT chest: HRCT for diffuse parenchymal disease
- CT abdomen: liver metastases
- CT abdomen: urinary calculus
- CT abdomen: appendicitis
- CT Colonography
- Cardiac CT: Calcium coronary scoring
Preliminary results from the first survey on CT were presented at the European Congress of Radiology in March 2015 (see Figures 5, 6 and 7).
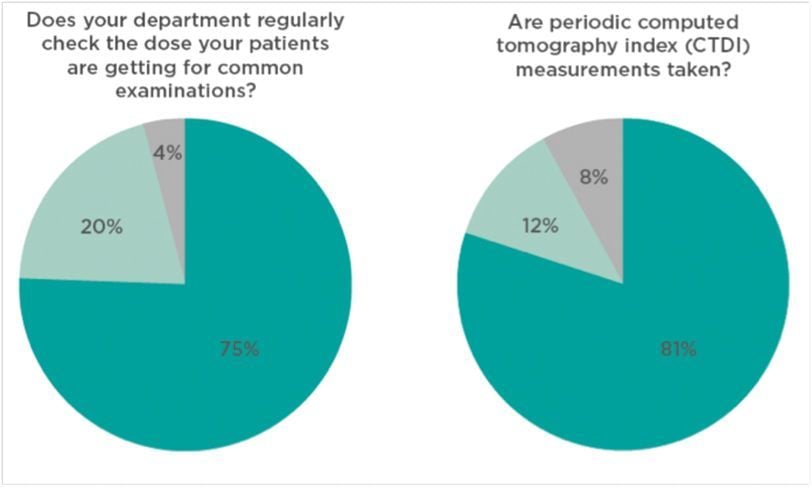
Figure 5. Head CT for Acute Stroke: Preliminary Results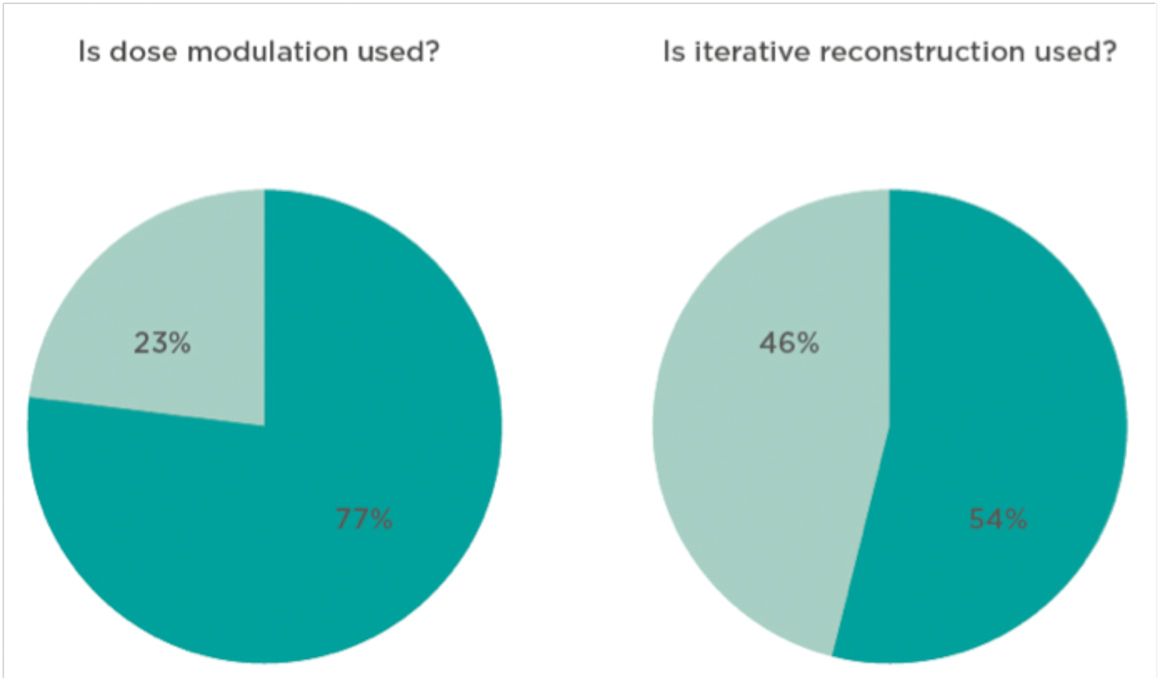 Figure 6. Head CT for Acute Stroke: Preliminary Results.
Figure 6. Head CT for Acute Stroke: Preliminary Results.
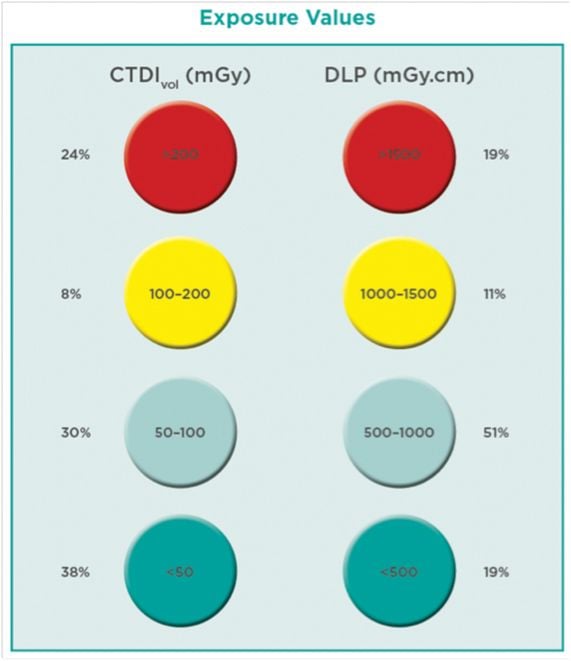
Figure 7. Head CT Practice: Preliminary Results
Even for this very simple examination 25% of doses reported are in the red circle, 25% in the green, and 50% in the middle. That means that even for simple examinations practice is very heterogeneous.
European Regulations
The European framework for quality and safety in imaging consists of the Euratom directive of 2013 (applicable from 2018) (Council Directive 2014), which outlines the principles of justification, optimisation, diagnostic reference levels, quality control, clinical audit and workers’ safety for x-ray imaging.
Safety guidelines for MRI are in preparation by the European Commission, in association with different stakeholders including radiologists, and will mainly cover workers’ safety. The European Medicines Agency has updated its guidelines for contrast agents used in imaging procedures (European Medicines Agency Committee for Medicinal Products for Human Use 2008).
Conclusion
The safe use of imaging should remain the main goal. However, quality of practice, organisation and management are absolutely essential for ensuring patient safety, which also implies a need for access to adequate IT tools. Benchmarking, clinical audit and patient information are also essential in this context and should be developed. Involvement of all stakeholders is crucial.
Note
‘Is your Imaging EuroSafe?’ comprises a series of monthly surveys on CT DRLs for different indications -
www.eurosafeimaging.org/survey
References:
Agency for Healthcare Research and Quality (n.d.) Patient safety indicators overview. [Accessed: 13 July 2015] Available from http://www.qualityindicators. ahrq.gov/modules/psi_overview.aspx
American College of Radiology (1998, rev. 2008) ACR position statement on quality control and improvement, safety, infection control, and patient education. [Accessed: 13 July 2015] Available from http://www.acr.org/~/media/ACR/Documents/ PGTS/PositionStatement.pdf
Brook OR, O’Connell AM, Thornton E et al. (2010) Quality initiatives: anatomy and pathophysiology of errors occurring in clinical radiology practice. Radiographics, 30(5):1401-10.
Cavanagh P (2015) A
new approach to clinical audit and safety by the ESR [abstract] [Accessed: 13
July 2015] Available from http://ipp.myesr.org/ ecr2015/index.php?p=startondemandaccess&mf-lid=
2144931f3fa7e17c130fa64c7005ee1f&sid=d658e 8ec7f687cad0a51061d63dd77c3
Council Directive (EC) 2013/59/EURATOM of 5 December 2013 laying down basic safety standards for protection against the dangers arising from exposure to ionising radiation, and repealing Directives 89/618/Euratom, 90/641/Euratom. [Accessed: 13 July 2015] Available from http://eur-lex.europa.eu/ legal-content/EN/ALL/?uri=CELEX:32013L0059
European Commission Directorate General for Energy (2014) Medical Radiation Exposure of the European Population, Part 1 & Part 2. Radiation protection 180. [Accessed: 13 July 2015] Available from https://ec.europa.eu/energy/sites/ener/files/documents/ RP180.pdf
European Commission Expert Panel on Effective Ways of Investing in Health (2014) Preliminary report on future EU Agenda on quality of health care with a special emphasis on patient safety. 10 July. [Accessed: 3 April 2015] Available from http://ec.europa.eu/health/expert_panel/opinions/ docs/005_safety_quality_of_care_en.pdf
European Medicines Agency Committee for Medicinal Products for Human Use (2008) Guideline on clinical evaluation of diagnostic agents. [Accessed: 13 July 2015] Available from http://www. ema.europa.eu/docs/en_GB/document_library/ Scientific_guideline/2009/09/WC500003580.pdf
European Society of Radiology (2014) Call for a European action plan for medical imaging to improve quality of care and patient safety. [Accessed: 13 July 2015] Available from https:// www.myesr.org/html/img/pool/ESR_2014_Call-for- Action-web.pdf
International Atomic Energy Agency (2014) Radiation protection and safety in medical uses of ionizing radiation. Draft safety guide DS 399. [Accessed: 13 July 2015] Available from http:// www-ns.iaea.org/downloads/standards/drafts/ ds399.pdf ; explanatory notes at http://www-ns. iaea.org/downloads/standards/note-verbales/ ds399-nv-english.pdf
Institute of Medicine (1999) To err is human: building a safer health system. [Accessed: 3 April 2015] Available from http://iom.nationalacademies. org/~/media/Files/Report%20Files/1999/To-Err-is- Human/To%20Err%20is%20Human%201999%20 %20report%20brief.pdf
Ip IK, Raja AS, Seltzer SE et al. (2015) Use of public data to target variation in providers’ use of CT and MR imaging among Medicare beneficiaries. Radiology, 275(3): 718-24.
Mandel C, Grimm J, Schultz T (2015) The Radiology Events Register (RAER): The role and value of incident reporting in radiology incident reporting. HealthManagement.org The Journal, 15(1): 109,112-4. Available from https:// healthmanagement.org/c/healthmanagement/ issuearticle/the-radiology-events-register-raer
National Patient Safety Foundation (2000) Agenda for research and development in patient safety. [Accessed: 31 March 2015] Available from http://c.ymcdn.com/sites/www.npsf.org/resource/ collection/4b2e552f-48fa-4dcf-8bd8-574ee15efd99/ Agenda_for_RD_in_Patient_Safety.pdf
Pennsylvania Patient Safety Authority (2011) Applying the universal protocol to improve patient safety in radiology services. Pa Patient Saf Advis, 8(2): 63-9. [Accessed: 13 July 2015] Available from http://patientsafetyauthority.org/ADVISORIES/ AdvisoryLibrary/2011/jun8%282%29/Pages/63.aspx
Ramanathan S, Ryan J (2015) Radiation awareness among radiology residents, technologists, fellows and staff: where do we stand? Insights Imaging, 6(1) :133-9.
Scott A (2007) Improving communication for better patient care. Radiol Technol, 78(3): 205-18. Woolf SH , Kuzel AJ , Dovey SM et al. (2004) A string of mistakes: the importance of cascade analysis in describing, counting, and preventing medical errors. Ann Fam Med, 2(4): 317–26.


