HealthManagement, Volume 17 - Issue 1, 2017
Globally Comparing Outcomes for Pulmonary Sarcoidosis
Pulmonologists have started comparing treatment outcomes for pulmonary
sarcoidosis patients among hospitals internationally. Participating hospitals
collect, exchange and discuss their outcomes with the aim to identify best
practices. The study was initiated by Prof. Dr. Jan Grutters, pulmonologist at
St. Antonius Hospital in the Netherlands. An international team of
pulmonologists was set up with leading experts regarding pulmonary sarcoidosis.
In addition to St. Antonius, five other centres of expertise are participating:
the Cleveland Clinic (USA), the Erasmus Medical Center (Netherlands), Royal
Brompton Hospital (UK), the University of Cincinnati Medical Center (USA) and
the University Hospital Leuven (Belgium).
Sarcoidosis is a rare immune disease involving the lungs and thoracic lymph nodes in approximately 90 percent of patients. Severe fatigue is one of the most common complaints in this patient group. No cure exists and treatment aims mostly for symptom relief and suppression of inflammation. As sarcoidosis is a rare disease, limited data is available—for instance from randomised clinical trials (RCTs)—on the effect of treatments. Pulmonologist Daniel Culver from the Cleveland Clinic sees value in collaborative efforts. “Assessing quality in complex chronic diseases has been elusive in many cases, especially when there is significant heterogeneity among patient phenotypes, lack of evidence to guide care pathways, and imprecise techniques to measure disease burden,” he says. “Process measures rather than outcome measures, may be easy and tempting to collect, but do not necessarily provide true insights into value differences between competing therapeutic strategies or medical systems. For pulmonary sarcoidosis, an orphan disease, it is especially important that collaborative efforts be at the forefront of defining expected outcomes and responsiveness to change of the various proposed clinical indicators. Since there is significant population heterogeneity and different approaches to therapy, pooling multiple centres provides added power and better capacity to compare the value of techniques.”
The project is the first example of an international implementation of value-based healthcare (VBHC). Based on transparently sharing treatment outcomes of routine clinical care, the hospitals can learn from each other and improve patient value—defined as outcomes relative to costs (Porter et al. 2013; 2016). Moreover, these results provide valuable input for further scientific research. As stated by Prof. Dr. Wim Wuyts, pulmonologist at the University Hospital Leuven: “It is very important for rare diseases that large centres bring together their experience. When evaluating a larger patient group, it is possible to significantly improve the diagnostics and the treatment of these complex conditions in the future.” The project started in 2014 with defining an international standard set of treatment outcomes. In 2015- 2016, data on these outcomes were collected retrospectively and first results were discussed in September 2016. Discussion of outcomes by the participating hospitals has already led to relevant insights on treatment differences between centres internationally.
Pulmonary Sarcoidosis
Sarcoidosis is a rare disease that can have high impact on the patient’s quality of life. It is a granulomatous disease of unknown aetiology, most commonly affecting young and middle-aged adults. Spontaneous remissions occur in about two-thirds of patients, but the disease course is chronic in 10 to 30 percent of patients (Costabel et al. 1999). Incidence and prevalence rates reported in the literature vary globally. Prevalence shows differences over geographical regions as well as ethnic groups, with the highest sarcoidosis prevalence reported in the Nordic countries and in African-Americans (Milman and Selroos 1990; Costabel et al 1999). Sweden has a prevalence of 160 per 100,000. Moreover, the incidence of sarcoidosis studied during the period 2003 to 2012 in Sweden was 11.5 per 100,000 per year and ranged by -10 percent to 30 percent, depending on the exact definition of the disease (Arkema et al. 2016). Sarcoidosis-related mortality is reported to be up to 7.6 percent in a US based population (Swigris et al. 2011). Most deaths are due to pulmonary fibrosis, neurologic and cardiac involvement (Costabel et al. 1999).
Patients suffer from a broad range of nonspecific symptoms, showing high variability regarding the inflammation level. Sarcoidosis is a condition that can occur anywhere in the body. In more than 90 percent of cases, sarcoidosis affects the lungs (Baughman et al. 2012). Severe fatigue is one of the most common complaints in this patient group (de Kleijn et al. 2009; Drent et al. 2012).
Treatment of Pulmonary Sarcoidosis
Despite advances since sarcoidosis was first described, much remains unknown regarding the aetiology of the disease. Due to the heterogeneous clinical manifestation, treatment can vary from no treatment to thorough follow-up with a variety of medications. The appropriate treatment for pulmonary sarcoidosis patients has not been well defined (Costabel et al. 1999). As stated in the Sarcoidosis Statement Committee, the symptoms that need corticosteroid therapy remain controversial. In order to tailor treatment well to the individual level, the availability of centre-specific outcome data has the potential to provide important advantages for quality improvement efforts.
Dr. Elizabeth Renzoni, pulmonologist at the Royal Brompton Hospital, adds that "This unique project provides the opportunity to compare diagnostic and management modalities between different international expert centres, anchoring these practices against outcome. For example, there are differences between the participating expert centres also in the use of certain diagnostic modalities such as PET scans to assess sarcoidosis activity, and it will be very interesting to assess how this impacts differences in corticosteroid use and outcomes. Because of the wide heterogeneity in presentation, organ involvement and disease severity, it is only through analysing outcomes in large groups of patients that the observed differences in diagnosis and management between expert centres can lead to improvements in patient care."
Definition of the Patient Population
The standard outcome set was designed specifically for pulmonary sarcoidosis patients. In order to be able to compare treatment outcomes among hospitals, not only the outcome measure and initial conditions need to be defined, but also a clear and uniform definition of the patient population itself is needed. The patient group is defined in line with the joint statement on sarcoidosis (Costabel et al. 1999). The international team acknowledges sarcoidosis is a very heterogeneous population. Therefore, it was decided there has to be pulmonary involvement, as 90 percent of sarcoidosis patients show pulmonary involvement. Specifically, the patient has to be:
- Diagnosed with pulmonary sarcoidosis
- The diagnosis has to be performed by a pulmonologist (based on the
joint statement on sarcoidosis)
- Extra-pulmonary sarcoidosis is not excluded
Standard Set of Outcomes
The pulmonologists from the six centres of expertise worked from early 2014 to early 2015 on the definition of an international standard set of outcomes measures for patients with pulmonary sarcoidosis. Prof. Robert Baughman, pulmonologist at the University of Cincinnati Medical Centre reports that “Value-based care has improved the outcome of patients with various conditions. Clinicians need better guidance on what to monitor as they follow their sarcoidosis patients. This initiative will collect information from centres across the world and determine the value of various markers of this multi organ disease.”
The team convened in a combination of face-to-face meetings, webinars and surveys. A systematic approach was used based on the principles of value-based healthcare (Porter 2010) and the methodology introduced in heart care by Meetbaar Beter (Meetbaar Beter 2013). First, a detailed description of the care delivery process for pulmonary sarcoidosis patients was created, providing an overview of ‘value’ created by all specialties involved throughout the entire care chain. Combined with a literature review, this was used to create a list of all potentially relevant health outcomes for pulmonary sarcoidosis patients. In order to end up with a concise and feasible set of outcome measures, the most important outcomes were prioritised anonymously by all team members based on three criteria: impact of the outcome on quality of life, impact of quality of care on the outcome and the number of patients affected by the outcome. As a result, the standard set combines both clinical outcomes, such as mortality, symptoms and side effects, with patient-reported outcomes, such as quality of life. Discussions were organised to reach consensus on the final outcome set. Following a similar process, the most relevant initial conditions were selected in order to allow corrections for the complexity of the population.
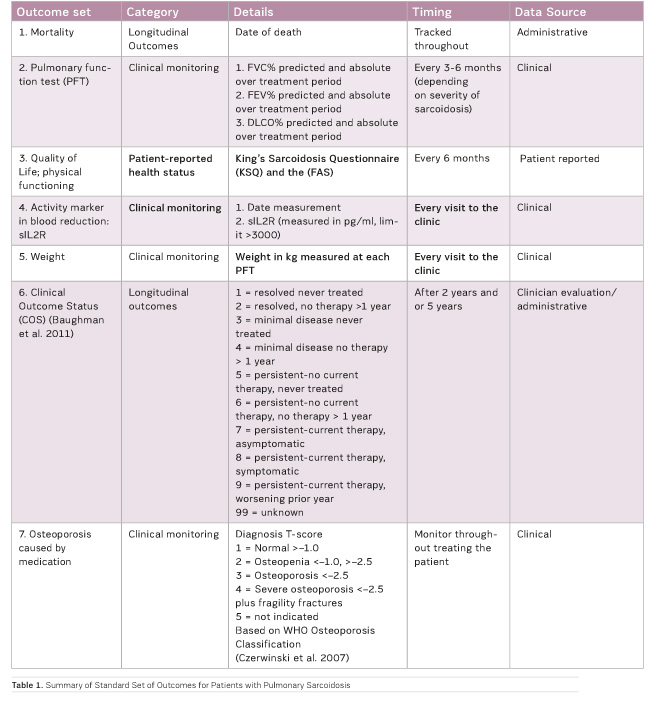
Standard Set for Pulmonary Sarcoidosis
A final standard set of 7 outcomes (Table 1) and 15 initial conditions
was identified. Some items from the final outcome set are not yet
systematically measured as a part of routine clinical care. For example, the
King’s Sarcoidosis Questionnaire (KSQ) (Patel et al. 2013) and the Fatigue
Assessment Scale (FAS), more often used for pulmonary sarcoidosis patients (de
Vries and Drent 2007), have been measured prospectively in each centre starting
early 2017.
“For pulmonary sarcoidosis, there is consensus on the standard set of
outcomes,” reports Frouke van Beek, pulmonologist at the St. Antonius Hospital:
"We not only measure the effects of treatment on, for example, lung function,
but also quality of life, patient's weight changes and whether osteoporosis
occurs as a result of certain medication. By sharing our data, we will be able
to learn what the best outcomes are of our treatment decisions, as there are
many difficult decisions to make during everyday care for our patients. This
approach can give us pulmonologists important new information on best practices
in specialised centres around the world. Especially as sarcoidosis is an orphan
disease and is very heterogeneous it would take a long time for a single centre
to collect sufficient data from which reliable conclusions can be drawn."
First Result
Preliminary results were discussed in September 2016 and have already led to several hypotheses for improvements in care delivery, such as better monitoring and treatment of osteoporosis, an important and frequently occurring complication in patients with pulmonary sarcoidosis due to long-term and high dosage of corticosteroids. We also observed that in all centres it concerns an adipose patient group and in some centres obesity is very common. Further analysis on corticosteroid use and weight changes over time is ongoing. Dr. Marlies Wijsenbeek, pulmonologist at the Erasmus University Medical Center adds that “though one should be cautious with interpreting retrospective data, these data give insight in the differences in clinical practices and underline the heterogeneity of patients with sarcoidosis and their responses to therapy. It is unique that six centres of expertise transparently compare their practices and outcomes, and in the next prospective phase also importantly incorporate the patient’s perception of his or her health status. Not only will this hopefully result in better care for patients, but also the project may potentially generate new insights for future clinical trial design in sarcoidosis. In a disease with no cure and arguable long-term benefits of the current treatments, research is much needed.”
Conclusions
Applying value-based healthcare in pulmonary sarcoidosis is very promising. Even though the data needs to be further analysed, a first international implementation of VHBC has been realised. For the first time treatment outcomes for a specific disease have been measured and compared internationally for routine clinical care. Preliminary results have already lead to new insights with relevant impact on care delivery in the participating centres. CEO at the St. Antonius Hospital, Prof. Dr. Douwe Biesma, states that "measuring and improving patient-relevant outcomes is a central part of the strategy of the St. Antonius Hospital and the collaborating centres. The Dutch funding agency ZonMW has made this scientific research regarding the implementation of VBHC possible. For the current study we will be able to learn whether the VBHC approach is also applicable in an orphan chronic disease such as sarcoidosis."
Key Points
- First international implementation of VBHC
- Internationally comparing outcomes for pulmonary sarcoidosis patients
with colleagues in 6 clinics (USA, UK, NL, BE)
- Promising approach for a rare, and in some cases a chronic, patient
group in an area where there is a paucity of data from RCTs
Nynke Kampstra
Nynke Kampstra is a PhD student at St. Antonius Hospital
within the value-based healthcare team. Her research focuses on the
implementation of value-based healthcare for pulmonary sarcoidosis.
Paul van der Nat
Paul van der Nat is senior advisor at St. Antonius
Hospital. He is leading the value-based healthcare team at St. Antonius. This
team works on the practical implementation of VBHC supported by scientific
research on VBHC implementation in a wide range of disease areas.
References:
Arkema EV, Grunewald, J, Kullberg S et al. (2016) Sarcoidosis incidence and prevalence: a nationwide register-based assessment in Sweden, Eur Respir J, 48(6):1690-9.
Baughman RP, Lower EE, Gibson K (2012) Pulmonary manifestations of sarcoidosis. Presse Med, 41(6 Pt 2): e289-302.
Costabel U, Hunninghake G on behalf of the Sarcoidosis Statement Committee (1999) ATS/ERS/WASOG statement on sarcoidosis. Eur Respir J, 14(4): 735-7.
Czerwiński E, Badurski JE, Marcinowska-Suchowierska E et al. (2007) Current understanding of osteoporosis according to the position of the World Health Organization (WHO) and International Osteoporosis Foundation. Ortop Traumatol Rehabil, 9(4): 337-56.
de Kleijn WP, De Vries J, Lower EE et al. (2009) Fatigue in sarcoidosis: a systematic review. Curr Opin Pulm Med, 15(5): 499-50.
De Vries J, Drent M (2007) Quality of life and health status in sarcoidosis: a review. Semin Respir Crit Care Med, 28(1): 121-7.
Drent M, Lower EE, De Vries J (2012) Sarcoidosis associated fatigue. Eur Respir J, 40(1) 255-63.
Meetbaar Beter (2013) Meetbaar Beter boek [Accessed: 22 December 2016] Available from meetbaarbeter.com/wp-content/uploads/2013/12/MeetbaarBeter_Boek_Antonius_Compleet.pdf
Milman N, Selroos O (1990) Pulmonary sarcoidosis in the Nordic countries 1950-1982. II. Course and prognosis. Sarcoidosis, 7(2): 113-8.
Patel AS, Siegert RJ, Creamer D et al. (2013) The development and validation of the King's Sarcoidosis Questionnaire for the assessment of health status. Thorax,
68(1): 57-65.
Porter ME (2010) What is value in health care? N Engl J Med, 363(26): 2477-81.
Porter ME, Larsson S, Lee TH (2016) Standardizing patient outcomes measurement. N Engl J Med, 374(6): 504-6.
Porter ME, Pabo EA, Lee TH (2013) Redesigning primary care: a strategic vision to improve value by organizing around patients' needs. Health Aff, 32(3): 516-25.
Swigris JJ, Olson AL, Huie TJ et al. (2011) Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med, 183(11): 1524-30.








