ICU Management & Practice, Volume 24 - Issue 1, 2024
Haemoadsorption may represent the new frontier in extracorporeal blood purification. Haemoadsorption has demonstrated effective extraction of a variety of toxins and drugs during episodes of sepsis, acute kidney injury or intoxication. Selective haemoadsorption can be performed by endotoxin-binding polymyxin B functionalised polystyrene fibres. The clinical application of non-selective haemoadsorption in sepsis or other conditions with an evident cytokine release syndrome presents a clear rationale.
Current Challenges
Critically ill patients present several challenges due to multiple organ involvement, complex clinical scenarios, increasing incidence of sepsis, and unmet diagnostic and therapeutic needs (Ronco et al. 2019; Clark et al. 2017). This determines a high demand for innovation and new paradigms. In the areas of sepsis and acute kidney injury (AKI), innovation is mandatory to improve current extracorporeal blood purification techniques (EBP), implement recent advances in technology and biomaterials, and cope with financial constraints. New areas of research include biomaterials, applied nanotechnology, microfluidics, new devices, new membranes and sorbents, transition from diffusion/convection to adsorption. Haemoadsorption (HA) may represent a new frontier in EBP (Ronco and Bellomo 2022). Solute mass separation may occur by barrier (membrane) or by solid agent (sorbent). Diffusion is mainly used in haemodialysis (HD) to remove small solutes, but it becomes inefficient for molecules above 3-5 KDa. Convection is applied in haemofiltration (HF) and haemodiafiltration (HDF) to remove middle molecules up to 10 KDa using ultrafiltration and solvent drag. Still, clearance is limited by the permeability of the membrane (sieving). Even the most modern membranes cannot effectively remove uremic toxins or sepsis mediators with molecular weight above 10-15 KDa. Thus, HA represents a new and interesting option to overcome the limitations of dialysis techniques, providing the required removal of large-middle molecules (Ronco and Bellomo 2023a).
Evolution of Sorbents
Sorbents have been used for two centuries. Natural carbons and allumo-silicates (to exchange ammonium and calcium) were recently substituted by synthetic polymers, chemically modified to become haemocompatible (Ricci et al. 2022). This allowed the clinical application of HA. Sorbents can be natural (zeolites and porous carbons) or synthetic (polymers). They may present high or low-density pores with a macro-, meso- or microporous structure (pore size > 500 Å, 20-500 Å or < 20 Å respectively) (Figure 1).
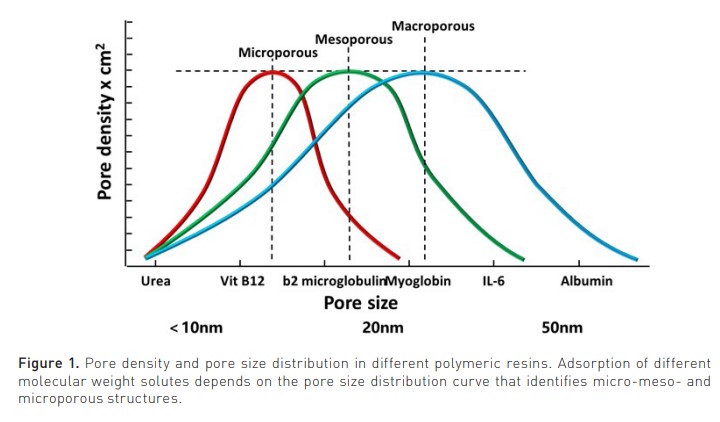
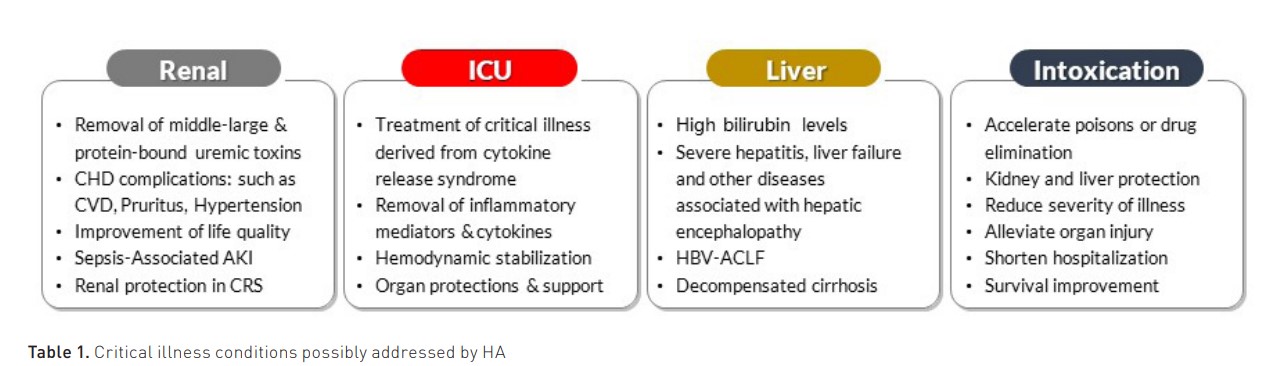
The pore density affects the amount of adsorbate (solute removed by the sorbent), while the pore size distribution affects the spectrum of molecules in the adsorbate. They may come in fibres, granules, beads, spheres, cylindrical pellets, flakes, or powder. They may operate by direct adsorption, anion or cation exchange, or immunoadsorption. Recently, the expanded capacity of adsorption by new polymeric resins has spurred new interest in the use of HA in several clinical conditions in intensive care (Table 1) (Copelli et al. 2023).
Rationale for Haemoadsorption
High levels of harmful molecules (solutes) in the blood are observed in the case of kidney or liver failure or in the case of exogenous intoxication and correlate with the severity of disease and mortality. This is the basis of life-saving treatments like EBP. However, the efficiency of toxin removal with current systems may be inadequate to fully replace kidney or liver failure function or to compensate for the dramatic surge in endogenous pro-inflammatory immune toxins as in the case of cytokine release syndromes (CRS). In those conditions where adequate blood purification cannot be effectively obtained, HA application finds a logical rationale as an alternative or additional therapeutic option (Ronco and Bellomo 2022).
Requirements for Safe and Effective Sorbent Therapy
Modern sorbent therapy requires an effective and haemocompatible sorbent material, a well-designed sorbent cartridge, and easy and simple implementation of related techniques with no or negligible side effects (Ronco and Bellomo 2023b).
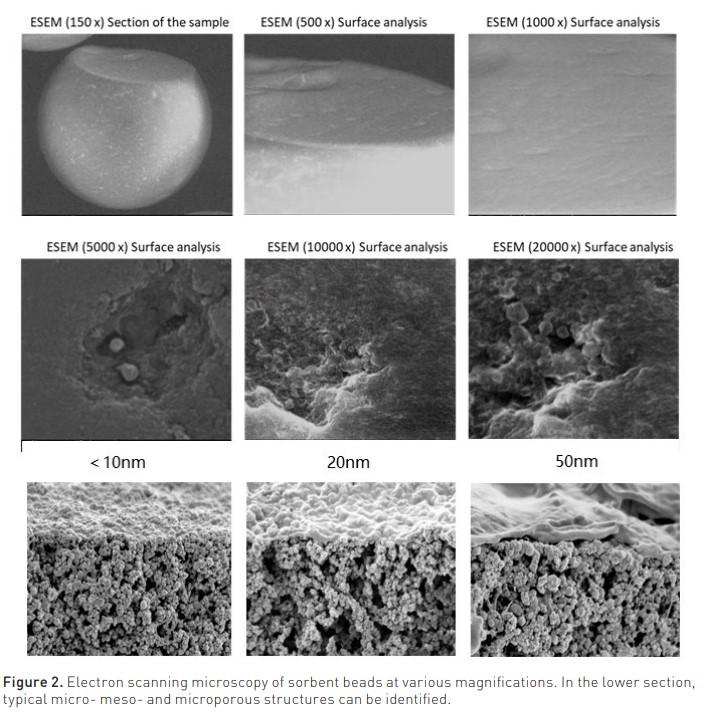
Modern sorbents are derived from polymers that create a porous structure with a high surface/volume ratio. Adsorption capacity is measured by experimental isotherms, specific curves that describe the maximal amount of solute that can be adsorbed by a unit of sorbent. Current sorbents demonstrate effective removal of molecules in the range between 10 and 55 KDa, including cytokines, chemokines, and protein-bound solutes, providing a new perspective on EBP. Porosity is regulated by the manufacturer, making it possible to target specific molecules. This results in a tridimensional sponge-like structure that can be analysed by electronic scanning microscopy (Figure 2). The forces involved in adsorption are 1) Hydrophobic bonds, generated by the hydrophobic affinity of the sorbent and the target molecules; 2) Ionic bonds, generated by electrostatic attraction between positively charged and negatively charged ions; and 3) Van der Waals forces, the interaction between electrons of one molecule and the nucleus of another molecule (Copelli et al. 2023).
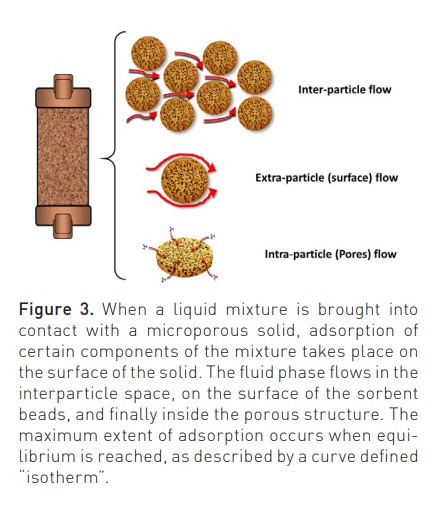
New sorbents are highly haemocompatible, as recently demonstrated in static and dynamic tests performed to detect possible monocyte activation during contact with human blood (Bellomo and Ronco 2023). Biocompatibility characteristics are further improved by a surface coating that improves interparticle blood rheology during treatment. The flow of the fluid phase (blood) inside the sorbent cartridge (divided into inter-particle, extra-particle and intra-particle) is governed by Darcy’s law and the Karman-Cozeny equation where the size of the beads, their porosity, the packing density, the length and diameter of the unit, the path tortuosity and Reynold’s number are the involved variables (Figure 3) (Ronco and Bellomo 2023c). Helical scanning techniques reported in Figure 4 (Lorenzin et al. 2019) demonstrate a homogeneous distribution of blood flow.
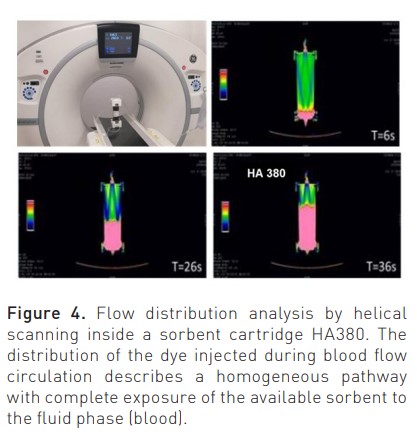
Molecular Targets: Selective and Non-Selective Haemoadsorption
Soluble mediators in the middle-large molecular weight range contribute significantly to organ injury, severity of disease and mortality in septic patients. As in the case of other clinical conditions derived from a cytokine release syndrome (infections, pancreatitis, burns, trauma, etc), sepsis mediators represent logical targets for removal by haemoperfusion. In sepsis, two approaches have been suggested: one based on selective targeting of a key molecule (e.g. endotoxin) and the other based on non-selective, broad molecular spectrum adsorption (Ronco and Bellomo 2022).
In case of the presence of the microbial agent, removal of circulating bacteria or viral particles can be obtained by special affinity binder cartridges. This treatment has been used in combination with special adsorbing membranes such as modified AN69 or adsorbing cartridges (Bellomo et al. 2024). In case of the presence of endotoxin in blood, detected by endotoxin activity assay, extracorporeal removal of endotoxin with polymyxin-B cartridge (PMX-HA) can be indicated alone or in conjunction with other adsorption or CRRT techniques (Kellum and Ronco 2023; Cruz et al. 2009; Dellinger et al. 2018; Klein et al. 2018). We should mention that different endotypes may also be a reflection of different conditions of the same patient in different time windows of the ICU stay. In these circumstances, the application of different adsorption and blood purification techniques can be time-sensitive, as suggested in a recent proposal for the sequential application of extracorporeal techniques (Ronco et al., 2023a).
On the other side, from selective potential, are non-selective sorbents capable of removing a broad range of molecules, including mediators and protein-bound solutes. Sepsis induces the expression of a dozen inflammatory mediators where no single molecule is responsible for the entire syndrome. In such circumstances, non-specific removal of the various mediators by haemoadsorption may represent the ideal condition to restore immune homeostasis (Ronco and Bellomo 2022; Ronco et al. 2023b). The cytokine release syndrome (CRS) is a systemic inflammatory response induced by bacteria, viruses, blood exposure to non-biocompatible materials, drugs, and antibody-based therapies or chimeric antigen receptor (CAR)-T cell therapy. Cytokines trigger a cascade with the activation of innate immune cells (macrophages and endothelial cells) with further cytokine release (Cobb and Lee 2021). The presence of a CRS may be demonstrated by biochemical measurements in the presence of the typical clinical picture characterised by hypotension and organ dysfunction. Therefore, it makes no sense to apply a cytokine removal technique if there is no evidence of systemic inflammation or elevated biochemical levels of cytokines. On the other hand, there is a specific time window for this type of intervention, which may prove beneficial in preventing the development of cytokine-mediated organ dysfunction or in protecting the kidney from disease and damage progression. Recently, special cartridges with microporous biocompatible resin have been made available with high capacity of cytokine adsorption (Bellomo and Ronco 2023). According to the peak concentration hypothesis (Ronco et al. 2004; Ronco et al. 2003), higher removal will occur for molecules with the highest concentration in blood and likely with the more impactful action on immuno-dysregulation. In such conditions, patients with impending or overt cytokine storm induced by different causes represent the ideal population for extracorporeal cytokine removal by haemoadsorption (Peng et al. 2008).
Adsorption Techniques and Relevant Indications
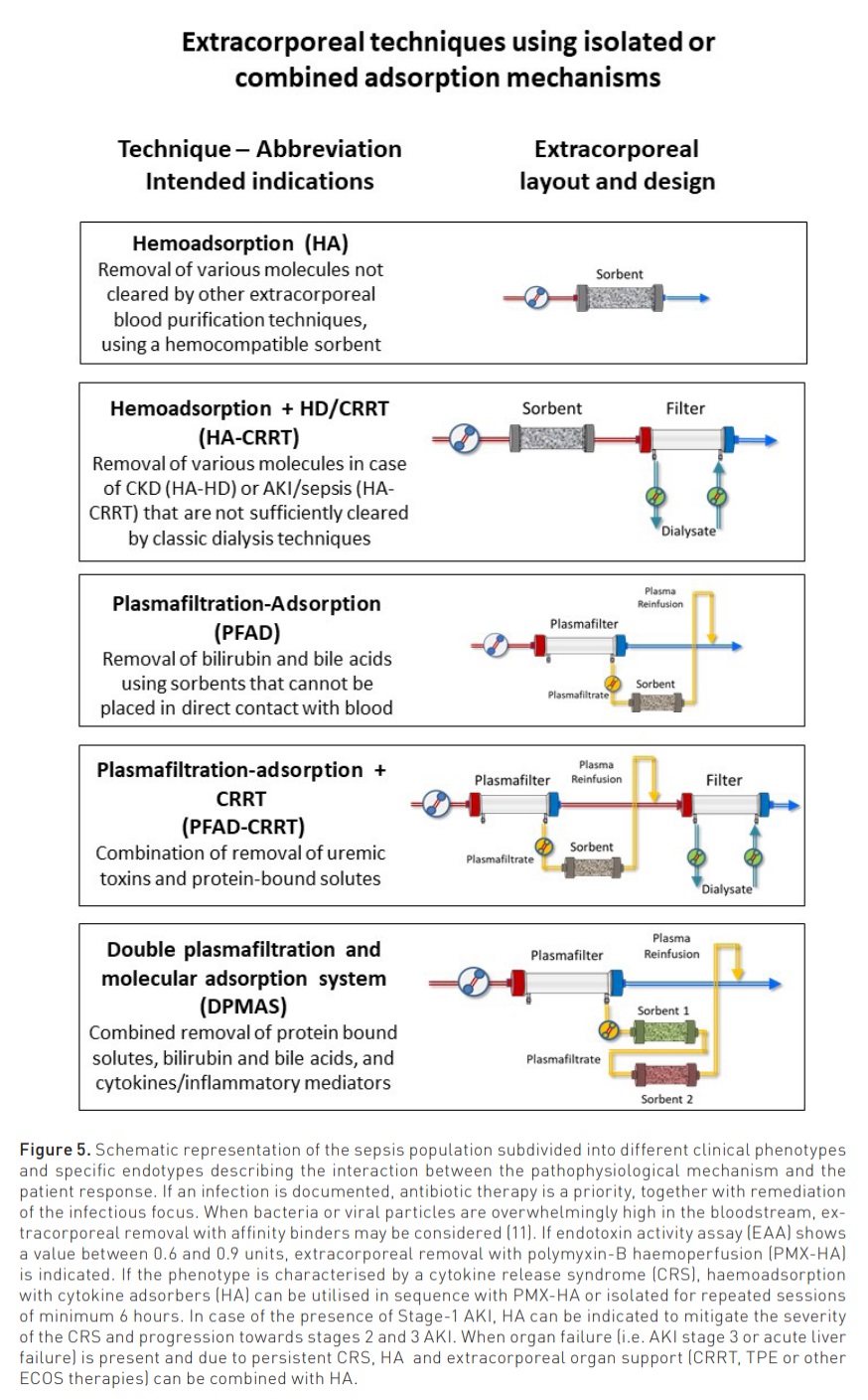
Modalities and indications for the clinical use of adsorption are reported in Figure 5 (Ostermann et al. 2023).
Haemoadsorption (HA): indicated in sepsis and other cytokine-release syndromes, intoxications and poisoning, the technique is generally applied for repeated sessions of 6 to 12 hours each with a blood flow between 100 and 300 ml/min.
Haemoadsorption + CRRT (HA-CRRT): indicated in sepsis-associated AKI for removal of cytokines and uremic toxins, this technique combines a sorbent cartridge in series with a CRRT filter. The complete saturation of the sorbent occurs between 6 and 12 hours.
Plasmafiltration-Adsorption (PFAD): This technique utilises a plasmafilter in the extracorporeal circuit. The filtered plasma, processed through a sorbent cartridge, is subsequently reinfused in the venous bloodline, reconstituting the whole blood. The technique is indicated to remove bilirubin, bile acids and other protein-bound solutes in acute liver failure or decompensated cirrhosis.
Plasmafiltration-Adsorption + CRRT (PFAD-CRRT): This technique combines PFAD with CRRT and is indicated to remove bilirubin, protein-bound solutes and uremic toxins in patients with combined liver and kidney failure.
Double plasmafiltration and molecular adsorption system (DPMAS): This technique utilises a plasmafilter in the extracorporeal circuit. The filtered plasma is processed through two different sorbent cartridges in series and is subsequently reinfused in the venous bloodline, reconstituting the whole blood. The technique is indicated in acute liver failure and decompensated cirrhosis to remove bilirubin, bile acids and other protein-bound solutes, together with circulating inflammatory mediators.
Haemoadsorption in ICU: Who is the Ideal Candidate?
Current EBP applied to AKI and septic patients presents important limitations (White et al. 2023). As such, new and more effective techniques are needed. Studies on the application of HA have been limited to small populations, producing sometimes controversial results (Zarbock et al. 2023; Zarbock et al. 2023b; Virág et al. 2021; Houschyar et al. 2017). This is in part due to the heterogeneity of the study patients. HA can, in fact represent an effective treatment for critically ill patients characterised by peculiar phenotypes (Figure 6). It is now evident that both sepsis and SA-AKI are a mixture of syndromes in which the cause and the response of the host play an important role in creating specific endotypes with peculiar characteristics and clinical pictures (Kellum and Ronco 2023; Cruz et al. 2009; Dellinger et al. 2018). Among them are haemodynamic instability, variations in temperature and leucocyte/platelet count, microthrombotic/microangiopathic profiles, and oliguria (Klein et al. 2018). In recent years, the possibility of measuring endotoxin activity using a specific assay (EAA) has allowed clinicians to identify a specific patient endotype in which endotoxin is detectable in blood and may represent a target for extracorporeal removal. Polymyxin-B-coated polystyrene fibres have been included in a special adsorption cartridge and are capable of removal of circulating endotoxin up to approximately 20 μg in a two-hour treatment (Cruz et al. 2009; Dellinger et al. 2018). Analyses have demonstrated that positive results are more likely in patients with significant organ failure and in patients with endotoxin activity between 0.6 and 0.9 (Klein et al. 2018). New studies should, therefore, be focused on this specific phenotype (Iba and Klein 2019).
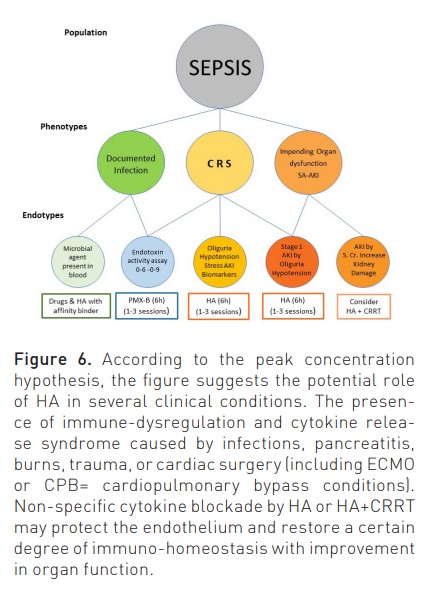
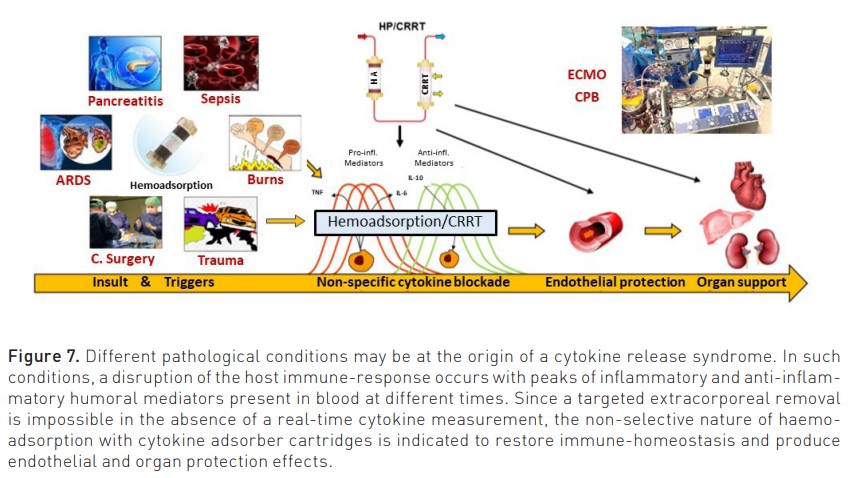
The same approach can be utilised to identify the right population affected by sepsis and/or sepsis-associated AKI. As described in Figure 7, sepsis and AKI are multifactorial syndromes, and only when presenting a specific endotype (Oliguria-based Stage 1 with sepsis or CRS-induced hypotension), there is a rationale for application of HA, even in the absence of any other RRT. In other stages, HA can be combined with CRRT if immuno-modulation or cytokine removal beyond renal support is desired (Zarbock et al. 2023b; Ronco and Kellum 2024).
Endpoints for Haemoadsorption Trials?
Once the right population has been identified, the endpoints for clinical trials should be clearly defined with a hierarchy of importance. For extracorporeal therapies such as HA, a progression from biochemical endpoints (removal of molecules) to biological endpoints (cellular effects of molecule removal such as enzymatic reactions, cellular functions, and immunological response) would be appropriate. Furthermore, pathophysiological endpoints (life parameters such as blood pressure, heart rate, PaO2/FiO2, diuresis, cardiac output, etc.) and then clinical endpoints (clinical outcomes including organ function and disease severity, need for dialysis, hospital-free days, mechanical ventilation free days, survival) should be considered. Survival represents the ultimate endpoint, but while trying to achieve sufficient evidence for this measure, it is important to avoid dismissing a treatment as ineffective because the right study has not yet been conducted. Evidence is a wall, and every small brick represents an addition to current knowledge. To advance in the acquisition of new evidence, it is important to share consensus on a structured research agenda (Bellomo et al. 2023) and proceed with collaborative efforts to build registries, well-designed studies, and big data collection.
Available Clinical Data
Several studies have reported the use of HA in the setting of intoxication, poisoning or drug overdose (e.g. paraquat or organophosphates, mushroom-related toxins, pesticides, valproate, carbamazepine and other) (Ghannoum et al. 2014; Kawasaki et al. 2005). Studies report information concerning clearance and mass removal when sorbents are used in isolation or in series with continuous haemodialysis. Extraction rates vary from 20 to 90% (Baylis et al. 2022), and mitigation of severity of the clinical picture has been reported.
There is limited information on the use of HA and plasma-filtration adsorption for severe liver failure, even though there is a robust rationale for targeting ammonia or bilirubin in this setting (Kittanamongkolchai et al. 2017; Santoro et al. 2007). Few studies confirm the utility of the DPMAS in comparison to plasma exchange (Marcello and Ronco 2023; Guo et al. 2020; Wang et al. 2023), and a large study on more than 1400 patients seems to confirm these results (Chen et al. Ongoing trial).
Several trials have addressed the possible effectiveness of PMX-HA for the removal of endotoxin in patients with sepsis. The first trial was reported in 2009 under the name of the EUPHAS (Cruz et al. 2009). This study reported physiological advantages on blood pressure, gas exchange, and use of vasopressors with polymyxin B haemoperfusion. In addition, it found that polymyxin haemoperfusion decreased time to mortality. The second study was a multicentre randomised controlled trial of the early use of polymyxin B haemoperfusion in patients with septic shock due to peritonitis (Payen et al. 2015). A larger study was the EUPHRATES trial (Dellinger et al. 2018). This multicentre randomised controlled trial compared polymyxin B haemoperfusion to conventional therapy in 450 adult critically ill patients with septic shock and an endotoxin assay activity of 0.60 or higher in 55 North American hospitals. This trial found no survival advantage among all participants or in the pre-specified subgroup of patients with a multiorgan dysfunction score > 9, both on intention to treat analysis or pre-protocol analysis.
A subsequent post-hoc assessment of the EUPHRATES study, however, has been conducted with a focus on the specific group of patients without extreme endotoxemia (Klein et al. 2018). In this subgroup of patients, haemoperfusion with polymyxin B appeared to carry a survival advantage on time-to-event analysis. A new study is currently underway in patients with endotoxaemic septic shock called TIGRIS (Clinical Trials.gov identifier: NCT03901807). This is a prospective, multicentre, randomised, open-label trial of standard medical care plus the PMX cartridge versus standard medical care alone in subjects with endotoxaemia and septic shock. Subjects in critical care areas will be assessed for septic shock using known or suspected infection, multiple organ failure, fluid resuscitation and hypotension requiring vasopressor support as primary criteria. Subjects will meet all entry criteria for study if endotoxin activity is within the range of ≥ 0.60 to <0.90. This study is scheduled to recruit 150 patients.
HA with non-selective cartridges represents a form of generic anti-inflammatory/immunomodulation HA strategy and has been studied in sepsis in the form of case series and comparative studies (Nassiri et al. 2021; Boss et al. 2021; Paul et al. 2021; Alharthy et al. 2021; Brouwer et al. 2019; Schädler et al. 2017; Supady et al. 2021; Esmaeili Vardanjani et al. 2021; Sazonov et al. 2021; Huang et al. 2010; Huang et al. 2013; Chu et al. 2020; Sánchez-Morán et al. 2023; Lertussavavivat et al. 2023; Becker et al. 2023).
Typical observations in the majority of studies were a remarkable reduction in Interleukin-6, tumour necrosis factor-alpha and other cytokines, significant reduction of inflammatory biomarkers (biochemical endpoints), Improved HLA-DR expression and monocyte function (biological endpoints), improved haemodynamic stability with reduction of vasopressor requirement (physiological endpoint), improvement in SOFA and other severity scores (clinical endpoints) and, in some studies, improvement of survival (ultimate endpoint). Data need to be further confirmed in larger trials and selected populations with homogeneous endotypes (Bellomo et al. 2024).
The Haemoadsorption Research Agenda
The state of research in the field of haemoadsorption resembles that of continuous renal replacement therapy (CRRT) in the 1980s. Studies need to be done to establish the biological, physiological, and clinical effects of sorbent-based techniques. Just like diffusion and convection across semipermeable membranes have been extensively studied over a period of more than 50 years, the third approach of mass separation based on the utilisation of solid agents (sorbents) should be explored to expand the future options provided by EBP. First, such research should first focus on achieving a better understanding of the basic aspects of the adsorption process. We need to understand the basic properties of each sorbent material, the mechanisms of adsorption and the potential side effects, including the unwanted removal of protective solutes such as antibiotics or nutrients (Bellomo et al. 2024; Migliorini et al. 2018; Ronco et al. 2001; Godi et al. 2021). We, therefore, need to establish a well-structured research agenda, in particular, the need to identify patient’s endophenotypes that are likely to benefit from HA. We need to establish adequate dose, frequency, and criteria for HA application. We need to identify target molecules and biomarkers and find a way to perform effective biomonitoring; we need to identify adequate endpoints for clinical trials to establish solid evidence, and we need to consider potential side effects and contraindications for this therapy promoting a medical-industry alliance for the development of new and more safe and effective devices. Another area of research will be the thorough analysis of the cost-benefits of HA, possibly moving from a strict budget-oriented approach to a more ethically oriented strategy. All these steps will allow significant progress in the field for the benefit of the patients.
Conflict of Interest
None.
References:
Alharthy A, Faqihi F, Memish ZA (2021) Continuous renal replacement therapy with the addition of CytoSorb cartridge in critically ill patients with COVID-19 plus acute kidney injury: A case-series. Artif Organs. 45(5):E101-E112.
Baylis S, Costa-Pinto R, Hodgson S et al. (2021) Combined Hemoperfusion and Continuous Veno-Venous Hemofiltration for Carbamazepine Intoxication. Blood Purif. 51(9):721-725.
Becker S, Lang H, Vollmer Barbosa C et al. (2023) Efficacy of CytoSorb®: a systematic review and meta-analysis. Crit Care. 27(1):215.
Bellomo R, Marcello M, Ronco C (2023) Hemoadsorption: Research Agenda and Potential Future Applications. Contrib Nephrol. 200:262-269.
Bellomo R, Mehta RL, Forni LG et al. (2024) Hemoadsorption. Clin J Am Soc Nephrol.
Bellomo R, Ronco C (2023) Clinical Applications of Adsorption: The New Era of Jafron Sorbents. Contrib Nephrol. 200:25-31.
Boss K, Jahn M, Wendt D et al. (2021) Extracorporeal cytokine adsorption: Significant reduction of catecholamine requirement in patients with AKI and septic shock after cardiac surgery. PLoS One. 16(2):e0246299.
Brouwer WP, Duran S, Kuijper M, Ince C (2019) Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care. 23(1):317.
Chen J et al. (n.d.) Precise Profiling of Liver Disease Patients With DPMAS Therapy, Treating Optimal Patients and Achieving Hard Endpoint (PADSTONE Study). Available at www.trial.gov
Chu L, Li G, Yu Y et al. (2020) Clinical effects of hemoperfusion combined with pulse high-volume hemofiltration on septic shock. Medicine. 99:e19058.
Clark WR, Gao D, Neri M, Ronco C (2017) Solute Transport in Hemodialysis: Advances and Limitations of Current Membrane Technology. Contrib Nephrol. 191:84-99.
Cobb DA, Lee DW (2021) Cytokine Release Syndrome Biology and Management. Cancer J. 27(2):119-125.
Copelli S, Lorenzin A, Ronco C (2023) Chemical-Physical Mechanisms of Adsorption for Blood Purification. Contrib Nephrol. 200:8-16.
Cruz DN, Antonelli M, Fumagalli R et al. (2009) Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 301(23):2445-52.
Cruz DN, Perazella MA, Bellomo R et al. (2007) Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care. 11: R47.
Dellinger RP, Bagshaw SM, Antonelli M et al. (2018) Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients With Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA. 320(14):1455-1463.
Esmaeili Vardanjani A, Ronco C et al. (2021) Early Hemoperfusion for Cytokine Removal May Contribute to Prevention of Intubation in Patients Infected with COVID-19. Blood Purif. 50(2):257-260.
Ghannoum M, Bouchard J, Nolin TD et al. (2014) Hemoperfusion for the treatment of poisoning: technology, determinant of poison clearance, and application in clinical practice. Seminars Dial. 27: 350-361.
Godi I, Lorenzin A, De Rosa S et al. (2021) Vancomycin Adsorption During in vitro Model of Hemoperfusion with HA380 Cartridge. Nephron. 145(2):157-163
Guo X, Wu F, Guo W et al. (2020) Comparison of plasma exchange, double plasma molecular adsorption system, and their combination in treating acute-on-chronic liver failure. J Int Med Res. 48(6):300060520932053.
Houschyar KS, Pyles MN, Rein S et al. (2017) Continuous hemoadsorption with a cytokine adsorber during sepsis - a review of the literature. Int J Artif Organs. 40(5):205-211.
Huang Z, Wand S, Yang Z, Liu J (2013) Effect on extrapulmonary sepsis-induced acute lung injury by hemoperfusion with neutral microporous resin column. Ther Apher Dial. 17: 454-461.
Huang Z, Wang S, Su W, Liu Ji-Yun (2010) Removal of humoral mediators and the effect on the survival of septic patients by hemoperfusion with neutral microporous resin column. Ther Apher Dial. 14:596-602.
Iba T, Klein DJ (2019) The wind changed direction and the big river still flows: from EUPHRATES to TIGRIS. J Intensive Care. 7:31.
Kawasaki CI, Nishi R, Uekihara (2005) How tightly can a drug be bound to a protein and still be removal by charcoal hemoperfusion. In overdose cases. Clin Toxicol. 43: 95-99.
Kellum JA, Ronco C (2023) The role of endotoxin in septic shock. Crit Care. 27(1):400.
Kittanamongkolchai W, El-Zoghby ZM, Eileen Hay J et al. (2017) Charcoal hemoperfusion in the treatment of medically refractory pruritus in cholestatic liver disease. Hepatol Int. 11(4):384-389.
Klein DJ, Foster D, Walker PM et al. (2018) Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 44(12):2205-2212.
Lertussavavivat T, Srisawat N (2023) Hemoperfusion in COVID-19. Contrib Nephrol. 2023;200:192-200.
Lorenzin A, Neri M, de Cal M et al. (2019) Fluid Dynamics Analysis by CT Imaging Technique of New Sorbent Cartridges for Extracorporeal Therapies. Blood Purif. 48(1):18-24.
Marcello M, Ronco C (2023) Bilirubin Adsorption with DPMAS: Mechanism of Action and Efficacy of Anion Exchange Resin. Contrib Nephrol. 200:201-209.
Migliorini E, Weidenhaupt M, Picart C (2018) Practical guide to characterize biomolecule adsorption on solid surfaces (Review). Biointerphases. 13(6):06D303.
Nassiri AA, Hakemi MS, Miri MM et al. (2021) Blood purification with CytoSorb in critically ill COVID-19 patients: A case series of 26 patients. Artif Organs. 45(11):1338-1347.
Ostermann M, Ankawi G, Cantaluppi V et al. (2023) Nomenclature of Extracorporeal Blood Purification Therapies for Acute Indications: The Nomenclature Standardization Conference. Blood Purif.
Paul R, Sathe P, Kumar S et al. (2021) Multicentered prospective investigator-initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb) in patients with sepsis and septic shock. World J Crit Care Med. 10(1):22-34.
Payen D, Guilbert J, Launey Y et al. (2015) Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicentre randomized control trial. Intensive Care Med 41:975-984.
Peng ZY, Carter MJ, Kellum JA (2008) Effects of hemoadsorption on cytokine removal and short-term survival in septic rats. Crit Care Med. 36(5):1573-7.
Ricci Z, Romagnoli S, Reis T et al. (2022) Hemoperfusion in the intensive care unit. Intensive Care Med. 48(10):1397-1408.
Ronco C, Bellomo R (2022) Hemoperfusion: technical aspects and state of the art. Crit Care. 26(1):135.
Ronco C, Bellomo R (2023a) History and Development of Sorbents and Requirements for Sorbent Materials. Contrib Nephrol. 200:2-7.
Ronco C, Bellomo R (2023b) Extracorporeal Techniques Based on Adsorption: Nomenclature, Hardware, and Circuit Design. Contrib Nephrol. 200:66-73.
Ronco C, Bellomo R (2023c) The Process of Adsorption and Cartridge Design. Contrib Nephrol. 200:74-81.
Ronco C, Bellomo R, Kellum JA (2019) Acute kidney injury. Lancet. 394(10212):1949-1964.
Ronco C, Bonello M, Bordoni V et al. (2004) Extracorporeal therapies in non-renal disease: treatment of sepsis and the peak concentration hypothesis. Blood Purif. 22(1):164-74.
Ronco C, Brendolan A, Dan M et al. (2001) Use of sorbents in acute renal failure and sepsis. Contrib Nephrol. (133):180-93.
Ronco C, Chawla L, Husain-Syed F, Kellum JA (2023a) Rationale for sequential extracorporeal therapy (SET) in sepsis. Crit Care. 27(1):50.
Ronco C, Samoni S, Bellomo R (2023b) Hemoperfusion and Immunomodulation. Contrib Nephrol. 200:142-148.
Ronco C, Kellum J (2024) Which Patient Phenotype Is the Ideal Candidate for Hemoadsorption in Acute and Chronic Kidney Disease? Integrative Medicine in Nephrology and Andrology. 11(1):e00001.
Ronco C, Tetta C, Mariano F et al. (2003) Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs. 27(9):792-801.
Sánchez-Morán, F, Mateu-Campos ML, Bernal-Julián F et al. (2023) Haemoadsorption Combined with Continuous Renal Replacement Therapy in Abdominal Sepsis: Case Report Series. J. Pers. Med. 13, 1113.
Santoro A, Mancini E, Ferramosca E, Faenza S (2007) Liver support systems. Contrib Nephrol. 156:396-404.
Sazonov V, Abylkassov R, Tobylbayeva Z et al. (2021) Case Series: Efficacy and Safety of Hemoadsorption With HA-330 Adsorber in Septic Pediatric Patients With Cancer. Front Pediatr. 9:672260.
Schädler D, Pausch C, Heise D et al. (2017) The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS One. 12(10):e0187015.
Supady A, Weber E, Rieder M et al. (2021) Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med. 9(7):755-762.
Virág M, Rottler M, Ocskay K et al. (2021) Extracorporeal Cytokine Removal in Critically Ill COVID-19 Patients: A Case Series. Front Med (Lausanne). 8:760435.
Wang L, Xu W, Zhu S et al. (2023) Double Plasma Molecular Adsorption System with Sequential Low-dose Plasma Exchange in Patients with Hepatitis B Virus-related Acute-on-chronic Liver Failure: A Prospective Study. J Clin Transl Hepatol. 11(4):908-917.
White KC, Serpa-Neto A, Hurford R et al. (2023) Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med. 49(9):1079-1089.
Zarbock A, Koyner JL, Gomez H et al. (2023a) Sepsis-associated acute kidney injury-treatment standard. Nephrol Dial Transplant. 39(1):26-35.
Zarbock A, Nadim MK, Pickkers P et al. (2023b) Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol. 19(6):401-417.
Zhu G, Zhang J, Ye M et al. (2024) Effect of the combination of HA380 hemoperfusion with CVVHDF on inflammatory indices and microcirculation in early septic shock. Tropical Journal of Pharmaceutical Research. 23 (1): 183-190.




















