ICU Management & Practice, Volume 24 - Issue 1, 2024
Complex polypharmacy and pathophysiology are common in the intensive care unit (ICU). Medicines optimisation is essential to deliver safe, effective, and individualised pharmacotherapy. This is ideally performed by a specialised ICU pharmacist.
Introduction
Medicines are the most common intervention in healthcare and are a central component of the life-saving treatment offered in the intensive care unit (ICU) (NICE 2015). Safe and effective use of medicines requires three key elements: (1) a comprehensive clinical assessment of the patient; (2) knowledge of treatment options available and supporting evidence base; and (3) the skill to assess potential benefits of pharmacotherapy against toxicity and to tailor dosing in accordance with individual patient need. Without due diligence with respect to these elements, the risk of treatment failure and unintended harm is great (NICE 2015). The aim of this short review article is to describe a selection of the challenges of medicine use in the severely unwell ICU patient and describe how the skills of clinical pharmacists are best utilised, within the multidisciplinary team (MDT) to achieve medicines optimisation.
The ICU Patient
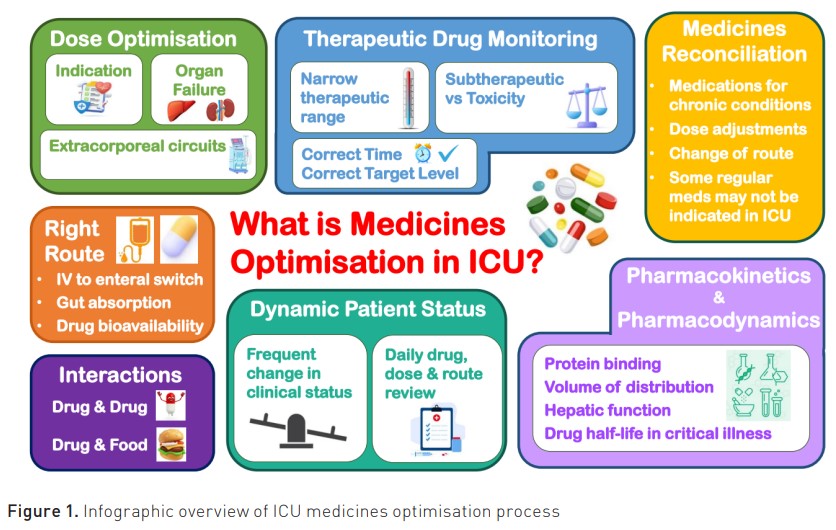
The ICU patient presents with unique pharmacotherapy challenges. These are broadly categorised under the headings of (1) medicines selection, (2) medicines administration and (3) medicines dosing. While there is increasingly more evidence from studies assessing treatments in critical illness, much of our existing knowledge of medicines is derived from phase one studies conducted in healthy adult patients. During critical illness, the ICU patient will develop altered physiology and organ dysfunction and receive many concomitant treatments (Hanks and McKenzie 2016). Furthermore, they present to the ICU with co-morbidities and polypharmacy. Therefore, research studies undertaken in healthy adults should be interpreted with caution. In selecting the optimum medicine, it is crucial to understand the limitations of the evidence, appraise and synthesise the pertinent resources and make an informed judgement as to the expected risks and benefits of treatment. ICU patients are also much more susceptible to adverse drug events than non-intensive care patients, further emphasising the importance of medicines optimisation (Devlin et al. 2010; Kane-Gill et al. 2010).
Medicines Administration
Medicines administration is challenging. The ICU patient is frequently mechanically ventilated and, therefore, cannot swallow; their medicines are typically administered intravenously (IV) or via an enteral feeding tube. The IV route provides rapid treatment and certainty of absorption. Prolonged use of IV formulations that contain adjuvants may, however, lead to increased exposure and toxic effects in some instances (e.g. SBECD with IV voriconazole) (Kiser et al. 2015). IV opioids and sedatives merit particular attention due to the risk of toxicity, physical dependence and iatrogenic withdrawal, which may ensue after as little as 3 to 5 days of treatment (McKenzie et al. 2023). Aside from the attendant toxicity risks, the process of preparing a medicine is also complex. Several steps are involved, including drug calculations, reconstitution, dilution and ensuring the concentration and administration rate are appropriate for the IV access available. In a U.K. study, 10.1% of IV medicine administrations were associated with error (Sutherland et al. 2010). This provides insight into the risks of IV medicines and is a stark reminder of the need for daily medicines review. Medicines are also administered orally or via enteral feeding tubes. Challenges in administration via the enteral route include suitability of formulation, interactions with enteral feeding and drug absorption/bioavailability (White 2015; Hanks et al. 2022).
Medicines Dosing
Finally, medicines dosing in the ICU is difficult. The ICU patient has complex pathophysiology; they may be hyperdynamic, hypotensive, fluid-overloaded and have end-organ dysfunction or outright failure. Extracorporeal devices may be required for organ support (e.g. renal replacement therapy, RRT or extracorporeal membrane oxygenation, ECMO). All of this impacts medicine’s absorption, distribution, metabolism and excretion, collectively known as pharmacokinetics (Hanks et al. 2022). Medicine or drug action (or pharmacodynamics) is impacted by reduced drug concentration at the receptor site and alteration in drug-receptor binding (Hanks et al. 2022). This requires expert consideration of patient, medicine and pathophysiology as well as awareness of the continually evolving ICU evidence base (McKenzie et al. 2024). It is beyond the scope of this article to describe extensively the impact of critical illness on pharmacodynamics and pharmacokinetics, but it is vital in clinical practice that issues are assessed carefully when deciding medicines dosing. If not, serum and tissue levels may not reach the desired target level, posing a risk of treatment failure. This has been described previously in the landmark beta-lactams study: defining antibiotic levels in intensive care patients (DALI-1) (Roberts et al. 2014). Where pharmacotherapy is concerned, a balance must be reached between maximising efficacy and minimising toxicity from adverse events (Bosma et al. 2018a). This is a core function of the specialist ICU pharmacist and is collectively termed medicines optimisation (McKenzie et al. 2024).
Here we describe the medicines optimisation process, highlight key references in its evolution and describe, with examples, how medicines optimisation occurs in ICU practice.
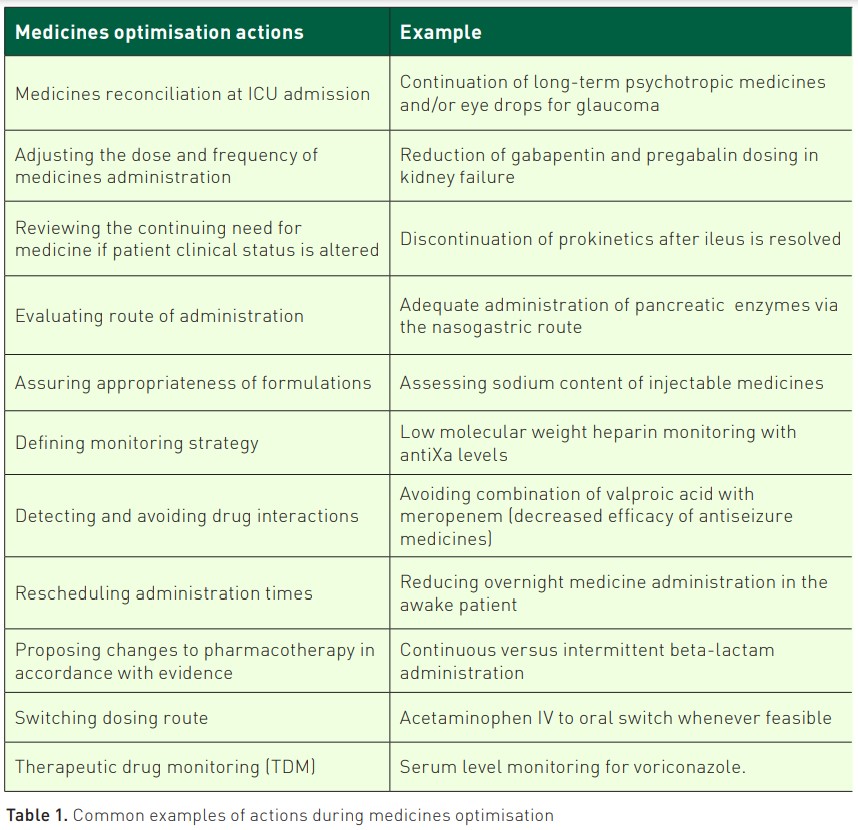
Structure of ICU Medicines Optimisation (Medicines Review)
In different parts of the world, the term medicines optimisation is interchangeable with other terms, including medicines review (Bosma 2019). For this article, we refer to it as medicines optimisation.Medicines optimisation is defined by the National Institute of Clinical Excellence (NICE) in NG5 2015 as "a person-centred approach to safe and effective medicines use, to ensure people obtain the best possible outcomes from their medicines” (NICE 2015). This involves performing a structured assessment to establish the safety and evaluate efficacy of medicines. The evidence base is applied to guide decisions about the care of the individual patient while assessing their needs, preferences, and values (Greenhalgh et al. 2014; Sackett et al. 1996). In ICU practice, this expert knowledge and skills are applied to interpret a patient’s clinical presentation, previous medical history, standard ICU observations (including mean arterial blood pressure, core temperature and urine output), fluid status and interpretation of standard and patient-specific pathology results, (e.g. kidney function and liver enzyme status). After the evaluation of relevant factors, each medicine is then optimised with the aim of maximising efficacy and minimising the risk of toxicity. Medicines optimisation can occur at any point during the ICU patient stay. Due diligence is given to optimisation at ICU admission and discharge. This is known as medicines reconciliation. Evidence shows a higher chance of error with medicines at ICU admission and/or discharge (Bosma et al. 2018b; Bourne et al. 2022). Medicines reconciliation also protects the patient from unintentional (dis)continuation of medication with high certainty of benefit, e.g. statins (Bell et al. 2011).
Frequency of Medicines Optimisation
Medicines optimisation should be delivered by an ICU specialist pharmacist, ideally daily, to account for rapidly changing ICU patient needs individually, although few ICUs have a 7-day clinical pharmacy service (Cheng et al. 2023). In ICUs, where a daily clinical pharmacy service does not exist, the authors recommend that ICU pharmacy professionals use a prioritisation tool to focus on patients with more complex medicines, e.g. Medication Related Complexity (MRC)-ICU (Sikora et al. 2022). Moreover, in this field of increasing complexity, the development of technical support through clinical decision support systems (CDSSs) is warranted since it supports the ICU specialist pharmacist and the MDT in medicines optimisation, e.g., in preventing administering high-risk medication combinations (Bakker et al. 2024).
Additional facets of medicines optimisation include exploring methods to deliver pharmacotherapy more effectively (e.g., multimodal analgesia) and supporting nursing colleagues’ workload by changing medicines administration (Devlin et al. 2018; Pearce and McKenzie 2023).
Landmark Publications
The introduction of the ICU specialist pharmacist typically begins with a focus on cost-saving and error reductions. The U.S., Australia and the U.K., amongst others, began this development more than 30 years ago (Dasta 1996; McKenzie 1996). In Europe, ICU specialist pharmacy is still very much in its infancy (Bosma et al. 2018a). Yet, in the ICU, there are the same challenges with the increasing complexity of polypharmacy and pathophysiology of the ICU patient.
By the early 2000s, the focus of the ICU specialist pharmacist had evolved from mainly reactive (i.e. reducing error after prescription) into a more proactive involvement in patient care, where medicines were ‘optimised’. In some countries (e.g. the U.K.), this resulted in much of the prescribing being delivered by specialist ICU pharmacists, as well as medical doctors, under the leadership of a consultant intensivist (Bourne et al. 2016).
In 2015, the PROTECTED-UK (Pharmacist’s Review and Outcomes: Treatment-Enhancing Contributions Tallied, Evaluated, and Documented) study was conducted in over 21 ICUs in the U.K. and published (Shulman et al. 2015). In this study, the investigators proposed definitions for clinical pharmacy activities as (1) medication error, (2) optimisation, or (3) consult. A medication error was defined as an error in the process of prescribing, dispensing, preparing, administering, monitoring, or providing medicine advice, regardless of whether harm has occurred. Optimisation was defined as a proactive contribution that sought to enhance patient care. A consult was defined as a reactive intervention in response to a request from a member of the MDT for an ICU specialist pharmacist review.
In PROTECTED-UK, the total number of interventions reported (over 2 weeks was 3294, 1693 (51.4%) were optimisations, 1393 (42.3%) were errors, and 208 (6.3%) were consults. Almost three-quarters of these (73.8%) were maximising efficacy and safety (Shulman et al. 2015).
In terms of time spent in ICU, specialist pharmacists spent 3.5 h per day (mean, ±SD 1.7) on direct patient care, reviewed 10.3 patients per day (±SD 4.2) and required 22.5 min (±SD 9.5) per review (Rudall et al. 2017). Intervention rate had a moderate inverse correlation with pharmacist time in the ICU (P = 0.05; r = 0.4). Optimisation rate had a strong inverse association with total number of prescriptions reviewed per day (p = 0.001; r = 0.7). A consultant C.P. had a moderate inverse correlation with number of errors identified (p = 0.008; r = 0.6) (Rudall et al. 2017). In 2023 (Cheng et al. 2023) reported pharmacist intervention over Saturdays and Sundays. Interestingly, out of 346 interventions, 166 (48.0%) were optimisations, 132 (38.2%) errors, and 36 (10.4%) consults. In terms of the overall patient benefit of pharmacist presence in ICU, conducting mainly medicines optimisations, Lee and colleagues reported an analysis of 13 observational and one randomised controlled trial that the inclusion of an ICU pharmacist in an ICU MDT was associated with lower mortality (odds ratio (OR), 0.78; 95% confidence interval (CI) 0.73–0.83) and reduced ICU length of stay (median reduction of 1.33 day; 95% CI − 1.75 to − 0.90) (Lee et al. 2019). This effect remained significant (OR, 0.79; 95% CI 0.64–0.97) after removing the largest non-randomised trial.
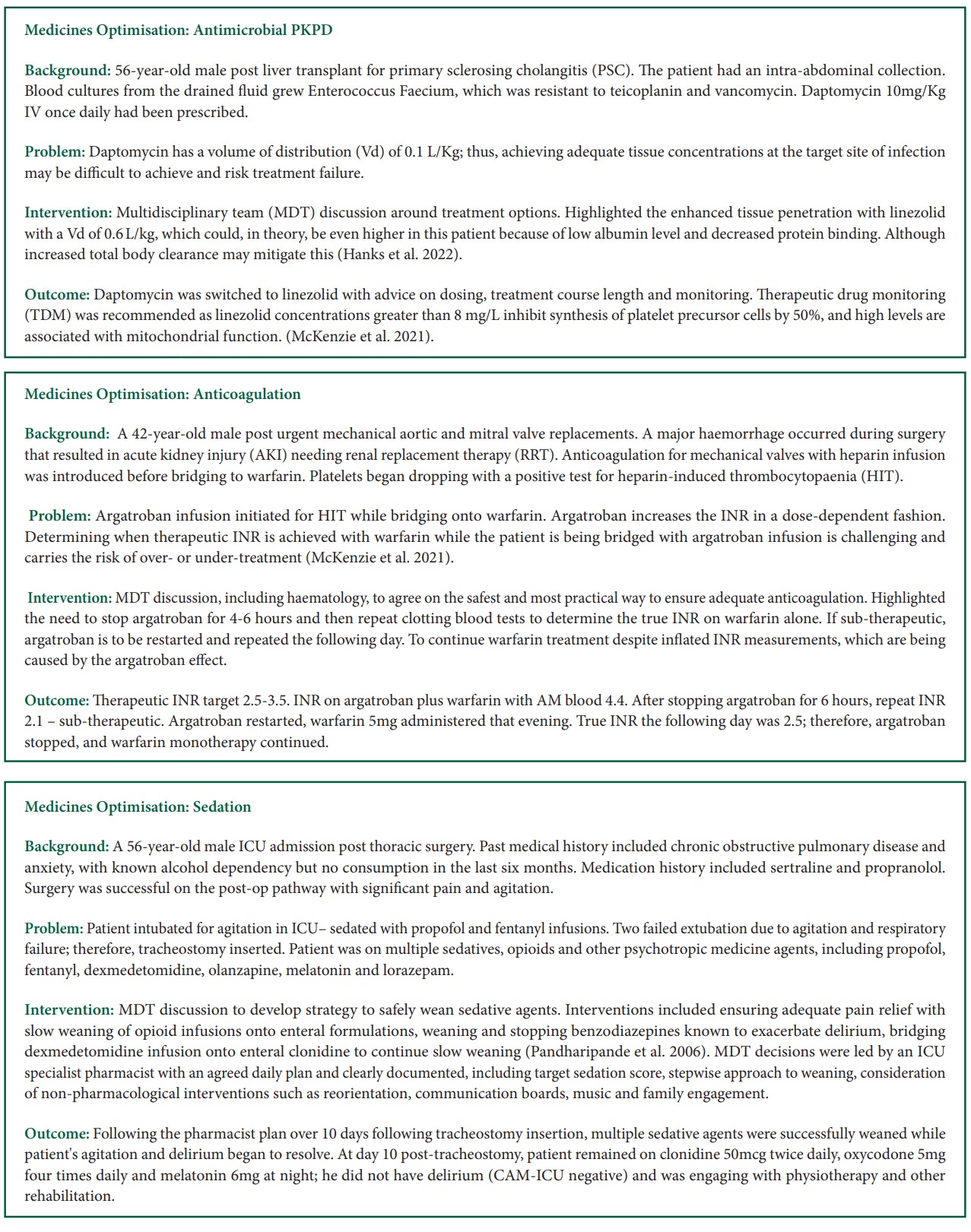
Conclusion
In ICU, polypharmacy is common. ICU patients are prescribed between 20 to 30 medicines per day. Some are essential for long-term co-morbidities encountered frequently in ICU, including type 2 diabetes and cardiovascular diseases. Others are essential ICU lifesaving therapy, e.g. vasopressors and antimicrobials. Adverse effects and drug interactions are common. Optimisation of these complex regimes is essential to protect our patients when they are severely unwell and to enhance their outcome.
Therefore, medicines optimisation performed by a specialist ICU pharmacist, ideally daily, is essential for optimum ICU clinical practice. In this article, we have described an outline of the medicines optimisation process, highlighted key references in its evolution and described, with several examples, how medicines optimisation occurs in ICU clinical practice.
ICU pharmacy professionals (specialist pharmacists and pharmacy technicians) provide patient care by focusing on medicines. They play a continuous and crucial role in medicines optimisation by ensuring the effective use of medicines through interactions with medical colleagues, nurses, allied health professionals, patients, and family members.
Conflict of Interest
Dr McKenzie declares an honorarium for her role as editor-in-chief of Critical Illness (www.medicinescomplete.com), published by Pharmaceutical Press. There are no further conflicts of interest.
References:
Bakker T, Klopotowska JE, Dongelmans DA et al., the SIMPLIFY study group (2024) The effect of computerised decision support alerts tailored to intensive care on the administration of high-risk drug combinations, and their monitoring: a cluster randomised stepped-wedge trial. Lancet. 403(10425):439-449.
Bell CM, Brener SS, Gunraj N et al. (2011) Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 306:840–847.
Bosma BE, van den Bemt P, Melief P et al. (2018a) Pharmacist interventions during patient rounds in two intensive care units: Clinical and financial impact. Neth J Med. 76:115–124.
Bosma L (2019) Medication Safety in Critically Ill Patients. Available at http://hdl.handle.net/1765/118754
Bosma LBE, Hunfeld NGM, Quax RAM et al. (2018b) The effect of a medication reconciliation program in two intensive care units in the Netherlands: a prospective intervention study with a before and after design. Annals of Intensive Care. 8:19.
Bourne RS, Baqir W, Onatade R (2016) Pharmacist independent prescribing in secondary care: opportunities and challenges. Int J Clin Pharm. 38:1–6.
Bourne RS, Jennings JK, Panagioti M et al. (2022) Medication-related interventions to improve medication safety and patient outcomes on transition from adult intensive care settings: a systematic review and meta-analysis. BMJ Quality & Safety. 31:609–622.
Cheng C, Walsh A, Jones S et al. (2023) Development, implementation and evaluation of a seven-day clinical pharmacy service in a tertiary referral teaching hospital during surge-2 of the COVID-19 pandemic. Int J Clin Pharm. 45:293–303.
Dasta JF (1996) Evolving role of the pharmacist in the critical care environment. Journal of Clinical Anesthesia. 8:S99–S102.
Devlin J, Skrobik Y, Gélinas CL et al. (2018) Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med. 46:1532-1548.
Evans L, Rhodes A, Alhazzani W et al. (2021) Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 47:1181-1247.
Greenhalgh T, Howick J, Maskrey N (2014) Evidence-Based Medicine Renaissance Group. Evidence-based medicine: a movement in crisis? BMJ. 348:g3725.
Hanks F, Phillips B, Barton G et al. (2022) How critical illness impacts drug pharmacokinetics and pharmacodynamics. Pharm J.
Ho K.M., Chavan S, Pilcher D (2011) Omission of Early Thromboprophylaxis and Mortality in Critically Ill Patients: A Multicenter Registry Study. Chest. 140:1436-1446.
Kiser TH, Fish DN, Aquilante CL et al. (2015). Evaluation of sulfobutylether-β-cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy. Crit Care. 19(1):32.
Lee H, Ryu K, Sohn Y et al. (2019) Impact on Patient Outcomes of Pharmacist Participation in Multidisciplinary Critical Care Teams: A Systematic Review and Meta-Analysis. Crit Care Med. 47:1243-1250.
McKenzie C, Spriet I, Hunfeld N (2024) Ten reasons for the presence of pharmacy professionals in the intensive care unit. Intensive Care Med. 50:147-149.
McKenzie CLR, Treacher D (1996) Impact of a specialist pharmacist on prescribing practice in the Intensive Care Unit. Intensive Care Med.
McKenzie C, Barton G, Phillips B eds. (2021) Critical Illness. London: Pharmaceutical Press.
National Institute for Health and Care Excellence (NICE) (2015) Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes.
Page V, McKenzie C (2021) Sedation in the Intensive Care Unit. Curr Anesthesiol Rep. 1-9.
Pandharipande P, Shintani A, Peterson J et al. (2006) Lorazepam Is an Independent Risk Factor for Transitioning to Delirium in Intensive Care Unit Patients. Anesthesiology. 104:21-26.
Pearce S, McKenzie C (2023) Antimicrobial preparation in the intensive care unit. Oh, what a waste. Intensive Crit Care Nurs. 77:103445.
Roberts JA, Paul SK, Akova M et al. (2014). DALI: defining antibiotic levels in intensive care unit patients: are current β-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. Apr; 58 (8):1072-83.
Rudall N, McKenzie C, Landa J et al. (2017) PROTECTED-UK - Clinical pharmacist interventions in the U.K. critical care unit: exploration of relationship between intervention, service characteristics and experience level. Int J Pharm Pract. 25:311-319.
Sackett DL, Rosenberg WM, Gray JA et al. (1996) Evidence-based medicine: what it is and what it isn't. BMJ. 312:71-2.
Sacks FM, Pfeffer MA, Moye LA et al. (1996) The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. N Engl J Med. 335:1001-1009.
Shulman R, McKenzie CA, Landa J et al. (2015) Pharmacist's review and outcomes: Treatment-enhancing contributions tallied, evaluated, and documented (PROTECTED-UK). J Crit Care.
Sikora A, Ayyala D, Rech MA et al. (2022) Impact of Pharmacists to Improve Patient Care in the Critically Ill: A Large Multicenter Analysis Using Meaningful Metrics With the Medication Regimen Complexity-ICU (MRC-ICU) Score. Crit Care Med. 50.
Sutherland A, Canobbio M, Clarke J et al. (2020). Incidence and prevalence of intravenous medication errors in the UK: a systematic review. Eur J Hosp Pharm. 27(1): 3-8.
Tran A, Fernando SM, Rochwerg B et al. (2022) Prognostic Factors Associated With Development of Venous Thromboembolism in Critically Ill Patients—A Systematic Review and Meta-Analysis. Crit Care Med. 50:e370-e381.
White RBV (2015) Handbook of drug administration via enteral feeding tubes.























