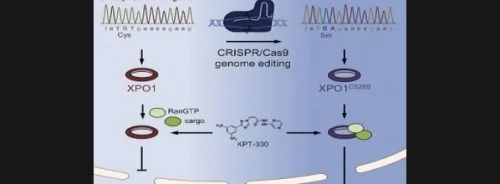
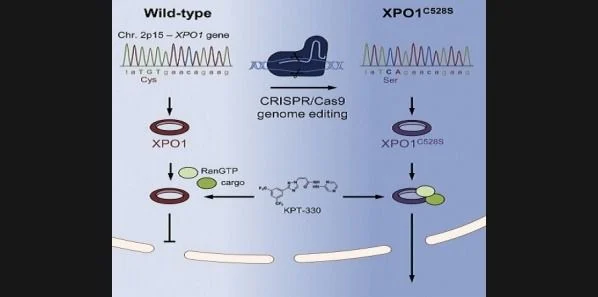
Genetically modifying cancer cells can aid in clarifying new cancer drugs’ mechanism of action, according to researchers at KU Leuven’s Laboratory of Virology and Chemotherapy (Rega Institute). In this new study, published online in Chemistry & Biology, the researchers confirm that KPT-330 molecule is able to target and block the Exportin-1 taxi (a key nuclear exporter protein) with extraordinary precision.
The researchers used a verification technique that was borrowed from virology, explains senior author Dirk Daelemans: “To develop a novel molecule into a drug, we need to first be able to verify that it targets exactly what we want it to target and nothing else. This is called drug-target validation. For antiviral drugs, drug-target validation is achieved through gene modification. But while it is relatively easy to genetically modify a virus, applying the same technique to potential anti-cancer drugs was nearly impossible – until now.”
In the human cell, the nucleus contains our DNA and acts as a ‘control centre’ while the cytoplasm – the compartment around the nucleus – acts as the cell’s ‘body’. It is here that proteins are produced and recycled. However, whether a protein is active or not depends on its location in the cell – either in the nucleus or in the cytoplasm.
The cell uses a transport system to ensure that a protein gets where it needs to be. In healthy cells, proteins are constantly being transported between the nucleus and the cytoplasm with the help of various transport proteins, such as Exportin-1 that transports more than 200 different proteins. Among Exportin-1’s passengers are tumour-suppressing proteins. When in the nucleus, these proteins are able to detect damaged DNA and trigger cell death.
Some cancers, however, disrupt Exportin-1’s normal functioning by transporting anti-cancer proteins out of the nucleus and into the cytoplasm – where they are prevented from carrying out their cancer-suppressing work.
KPT-330 Molecule: Inhibitor of Exportin-1
Researchers at the Rega Institute and the Department of Chemistry previously reported an inhibitor of the Exportin-1 taxi. Karyopharm Therapeutics Inc., a clinical-stage pharmaceutical company focused on discovery and development of novel drugs directed against nuclear transport targets, has identified and developed the advanced Exportin-1 inhibitor KPT-330 for the treatment of cancer.
The new research by Professor Daelemans and colleagues has demonstrated that gene modification enables drug-target validation and drug-target selectivity studies in cancer cells.
“We know that the molecule KPT-330 attaches to a particular amino acid, a building block of the Exportin-1 protein. Thanks to the latest developments in gene technology, we were able to modify that particular Exportin-1 amino acid in cancer cells,” explains Professor Daelemans.
“The result? The key no longer fit in the lock and KPT-330’s anti-cancer effect disappeared. This was the proof we needed to show that this molecule acts exclusively on the Exportin-1 taxi and no other targets. This technique can be used to develop other anti-cancer drugs as well, which bodes very well for the discovery and development of future cancer drugs.”
Source: KU Leuven
Image Credit: KU Leuven
The researchers used a verification technique that was borrowed from virology, explains senior author Dirk Daelemans: “To develop a novel molecule into a drug, we need to first be able to verify that it targets exactly what we want it to target and nothing else. This is called drug-target validation. For antiviral drugs, drug-target validation is achieved through gene modification. But while it is relatively easy to genetically modify a virus, applying the same technique to potential anti-cancer drugs was nearly impossible – until now.”
In the human cell, the nucleus contains our DNA and acts as a ‘control centre’ while the cytoplasm – the compartment around the nucleus – acts as the cell’s ‘body’. It is here that proteins are produced and recycled. However, whether a protein is active or not depends on its location in the cell – either in the nucleus or in the cytoplasm.
The cell uses a transport system to ensure that a protein gets where it needs to be. In healthy cells, proteins are constantly being transported between the nucleus and the cytoplasm with the help of various transport proteins, such as Exportin-1 that transports more than 200 different proteins. Among Exportin-1’s passengers are tumour-suppressing proteins. When in the nucleus, these proteins are able to detect damaged DNA and trigger cell death.
Some cancers, however, disrupt Exportin-1’s normal functioning by transporting anti-cancer proteins out of the nucleus and into the cytoplasm – where they are prevented from carrying out their cancer-suppressing work.
KPT-330 Molecule: Inhibitor of Exportin-1
Researchers at the Rega Institute and the Department of Chemistry previously reported an inhibitor of the Exportin-1 taxi. Karyopharm Therapeutics Inc., a clinical-stage pharmaceutical company focused on discovery and development of novel drugs directed against nuclear transport targets, has identified and developed the advanced Exportin-1 inhibitor KPT-330 for the treatment of cancer.
The new research by Professor Daelemans and colleagues has demonstrated that gene modification enables drug-target validation and drug-target selectivity studies in cancer cells.
“We know that the molecule KPT-330 attaches to a particular amino acid, a building block of the Exportin-1 protein. Thanks to the latest developments in gene technology, we were able to modify that particular Exportin-1 amino acid in cancer cells,” explains Professor Daelemans.
“The result? The key no longer fit in the lock and KPT-330’s anti-cancer effect disappeared. This was the proof we needed to show that this molecule acts exclusively on the Exportin-1 taxi and no other targets. This technique can be used to develop other anti-cancer drugs as well, which bodes very well for the discovery and development of future cancer drugs.”
Source: KU Leuven
Image Credit: KU Leuven
References:
Daelemans D, Neggers JE, Vercruysse T et al. (2015) Identifying Drug-Target Selectivity of Small-Molecule CRM1/XPO1 Inhibitors by CRISPR/Cas9 Genome Editing. Chemistry & Biology, January 2015. DOI:
http://dx.doi.org/10.1016/j.chembiol.2014.11.015
Latest Articles
Cancer, DNA, proteins, cell death, KPT-330, genetic modification
Genetically modifying cancer cells can aid in clarifying new cancer drugs’ mechanism of action, according to researchers at KU Leuven’s Laboratory of V...